UNEC Journal of Engineering and Applied Sciences Volume 5, No 1, pages 26-34 (2025) Cite this article, 1294 https://doi.org/10.61640/ujeas.2025.0503
The current trend in the use of insulating coatings to protect pipelines from soil corrosion is the use of pipes with factory applied protective coating. The technology of applying insulating materials in factory conditions allows to obtain high-quality safe coating, which service life equals to the service life of the pipe metal.
However, the direction of application of trace insulation is currently also relevant. The main task of application of this kind of coatings is to ensure high adhesion and other performance characteristics close to the factory applied coatings. Initial values of adhesion to the metal surface and its stability over time significantly affect the reliability and safety of the operated pipeline [1].
Widely used coatings in trace conditions are polymer tapes with sticky adhesive and bitumen-mastic layer.
The disadvantage of adhesive polymer tapes is the instability of adhesion to metal. In 5 years after application adhesive strength in many cases completely disappears due to destruction of thin (up to 100 microns) adhesive layer and the metal is unprotected from corrosion. Loss of adhesion is promoted by electrochemical processes at the metal-coating contact, which also lead to degradation of the adhesive layer. A serious disadvantage of polymer tapes is also the formation of hipped voids in the near-seam zone, which later become corrosion centers [2].
Bitumen-polymer tapes in comparison with sticky polymer tapes have a number of advantages. Tapes based on bitumen-polymer materials have a greater thickness of the mastic layer (more than 1.0 mm), which eliminates the formation of tent voids. High wetting ability of these tapes allows to maintain adhesion over time and provide resistance to cathodic peeling [2].
However, the adhesion and other performance characteristics of bitumen-polymer and sticky polymer tapes are not enough for reliable operation of main pipelines [3].
The search for new insulating materials, the study of the principles of adhesion formation on the metal surface and its improvement is an urgent problem today.
Currently, Russia produces anticorrosive insulating material asmol and roll insulation materials based on it. The peculiarity of asmol is that it has additional chemical adhesion to metal, which allows to extend the service life of the protective coating and, consequently, of the pipeline itself [4].
The authors faced the task of studying the regularity of adhesion of asmol materials in the process of its production and operation in real conditions.
In the article adhesion properties of innovative insulating material - asphalt-resin oligomer (asmol) used for anticorrosion protection were studied. Special attention was paid to the mechanism of formation of adhesive bond between asmol and metal surface.
The technique of asmol synthesis based on the interaction of heavy oil residues with monomers in the presence of sulfuric acid as a catalyst is described. The correlation between synthesis stages and adhesion ability of the material is established. The stability of adhesion for industrial samples obtained in laboratory conditions, as well as their efficiency in real conditions of pipeline operation was experimentally confirmed [5].
The metallographic method – light (optical) microscopy method was used to analyze the interaction of asmol with metal.
High results of adhesion testify to chemical interaction of asmol components with metal, which confirms the put forward hypothesis.
2.1 Materials
Asmol material is a product of petrochemical synthesis - interaction of heavy oil residues (tar deasphalting asphalt) with cube residues of isoprene production (di- tri- n-mers of isoprene) in the presence of sulfuric acid.
2.2 Asmol synthesis
Asmol synthesis consists of several stages. In the first stage of the process, heavy oil residue – asphalt of deasphaltization of tar is mixed with the cube residue of isoprene production (di- tri- n-mers of isoprene) at 120-125℃. Then in the next stage, 96% H2SO4 is added slowly at 150℃ for 400 minutes without foaming, which plays the role of catalyst and sulfating agent at the same time. The third stage, product stabilization (400-800 min), ensures the complete consumption of sulfuric acid [5].
Component ratio:
The finished product, asphalt-resin oligomer asmol, has an aqueous draw close to neutral, i.e. it does not contain free sulfuric acid. As a result of the chemical reaction, two types of organic acids are formed in asmol - strong sulfonic acids and weak carboxylic acids. According to the IR-spectroscopy data the carbon groups of aldehydes, ketones and SO2 group in sulfates, which can be part of acids, as well as be functional groups of neutral resins and asphaltenes, were also identified. Non-polar components of asmol are petroleum hydrocarbons [6].
During asmol synthesis, samples were taken and the adhesion to metal of the resulting intermediates over time at various stages of production was determined.
To evaluate the adhesion properties of asmol and its intermediates during synthesis, samples in the form of mushrooms made of grade 3 and 15 steel were used according to the method of GOST 26589 [7]. The obtained intermediates were applied to the surface of the steel moulds at a temperature of 80℃ and then kept at this temperature for 20 minutes under a press with a pressure of 15 kg/cm2 (figure 1).

Figure 1. Schematic diagram of the sample for determining the adhesion of asmol and its intermediates: 1 - layer of ASMOL or semi-product; 2 - place of attachment of the tensile machine.
After the steel moulds cooled to room temperature, at least 5 specimens were tested on a tensile testing machine. The speed of the machine grippers was 5 mm/min.
Instron type machine with ultimate load of 10 kN was used as a tensile machine.
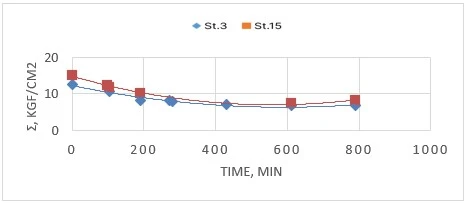
Figure 2. Changing the adhesion of ASMOL to the metal surface substrate during the process of its preparation
Figure 2 shows that the nature of the dependence of the adhesion value on the duration of synthesis is determined by the grade of steel used in the manufacture of the substrate. The highest adhesion values were obtained to the substrate made of high-carbon structural steel grade «St.15» (carbon content 0.15%), to low-carbon steel grade «St.3» (carbon content 0.03%) adhesion of asmol and its intermediates is less.
One possible explanation for the decrease in adhesion in intermediate samples could be the following. In the process of production not all the added acid has time to react by the given moment of time. The acid that has not had time to react and is in the sample interacts with the metal of the substrate to form a fragile film of ferrous sulfate. The formation of the latter reduces the adhesion of asmol to the substrate. When the reaction mass is heated at the stabilization stage, excess sulfuric acid reacts with the components of asmol: the adhesion strength of asmol samples taken at this stage increases.
The difference in the adhesion of ASMOL to high-carbon and low-carbon steel is probably due to the size of iron crystalline grains. An increase in the size of the crystal lattice can lead to an increase in the available surface atoms, which are active centers for the chemical reaction. The larger the size of iron crystals, the faster the formation of iron sulfate [7].
At full completion of the technological process (up to 10 hours), at pH of the finished product 6.0-6.5 stabilization of the adhesion index of the finished asmol is observed.
On the basis of petroleum polymer asmol tape insulation materials LIAM and ARMAS, as well as asmol primer are produced [8].
LIAM tape is a polymer tape with a mastic layer applied on the basis of «Asmol». Polyvinylchloride or polyolefin tapes are used as a tape base. To prevent sticking of the mastic layer in the roll, an anti-adhesive coating is applied on top of it [8, 9].
LIAM tape is available in several variants, including the following grades:
LIAM tape has different width range: 450, 225, 150, 112 and 90 mm. It is supplied to the route in the form of rolls.
Asmol roll material reinforced with glass mesh ARMAS is used as an auxiliary material in the structure of external anticorrosion coatings in order to strengthen them. ARMAS is produced by combining the melt of asmol oil polymer with reinforcing glass mesh with mesh sizes 2,5x2,5; 3,4x3,4 mm or similar. Depending on the conditions of application ARMAS as well as LIAM is produced in several variants, summer, winter and heat-resistant [10].
The primer is a 30% solution of asmol oil polymer in an organic solvent containing aromatic hydrocarbons. Due to its low viscosity and good covering power, asmol primer prepares the surface to be protected well. Application of asmol primer does not require thorough, sandblasting cleaning of the surface.
The next stage is the study of adhesion properties of asmol materials during industrial production.
Let's consider the adhesion value of industrial batches of tape materials on the example of LIAM tape. This value is somewhat lower than that of the initial asmol, because the composition of asmol mastic for LIAM tape contains various technological additives to simplify the technology of application and production [11].
Table 1 shows adhesion characteristics of industrial batches of LIAM tape measured by the method according to GOST R 51164 [12].
As follows from the data in the table, the stability of adhesion characteristics of different grades of LIAM tape is observed during the production process.
The next stage was to study the changes in adhesion of asmol coatings with the use of LIAM tape during operation in real highway conditions.
Figure 3 shows the appearance of the asmol coating after 6 years of operation. The coating is well preserved, corrugations are absent.
The appearance of the coating is satisfactory.

Figure 3. Appearance of the coating insulation based on LIAM tape. Gas pipeline branch in Kamensk-Uralsky DN 700
The results of coating adhesion measurements are presented in table 2. Standard protective coating constructions used in pipeline insulation:
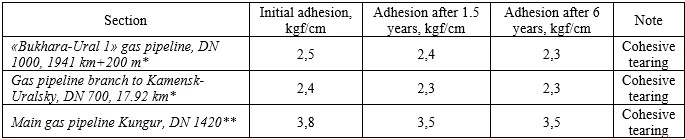
Table 2. Dependence of adhesion characteristics on trunk pipelines on operation time
*Coating construction – primer + LIAM + wrapping coat
**Coating construction – primer + ARMAS + LIAM + wrapping layer

Figure 4. Cohesive peel-off of the coating based on LIAM tape during peeling. Gas pipeline branch in Kamensk-Uralsky DN 700
A significant layer of asmol mastic remains on the metal, therefore, the adhesion strength of asmol mastic to metal is higher than its tensile strength. As a result of measuring adhesion according to the method according to GOST R 51164 we determine the value of cohesion, i.e. the strength of asmol mastic [13].
In accordance with the above, it was necessary to evaluate the actual adhesion and study its change over time.
To estimate the actual adhesion, i.e. the strength of adhesion of asmol mastic with metal, the following developed method was used.
Asmol-treated AB plates were glued to metal rods 7 mm in diameter with epoxy glue as shown in figure 5. Then tensile tests were performed using a universal dynamometer of «Instron» company. The accuracy of load determination was 0.05 N.
To ensure the alignment of the rods, a special slipway was made (figure 6), and a fluoroplastic insert was inserted in the center of the slipway with tension. A 7 mm diameter slot was milled in the insert, in which the rods were placed. The plates were placed in the slots in the slipway and the rods were glued to them. The fluoroplastic insert prevented the rods from gluing to the slipway. The plate cutouts in the slipway were made so that in one case the rods were perpendicular to the plates, and in the second case they were oriented at an angle of 45° to the plates. The sketch of the slipway is shown in figure 6.
Three identical samples were tested and fractured at loads Q = 71 N; 70.8 N; 70.9 N. The average breakaway stress amounted to 1.84 MPa. Moreover, the measurement results have practically no scatter. This is an order of magnitude higher than the required value according to GOST R 51164 (0.2 MPa).
After destruction of samples plates were subjected to metallographic studies by light (optical) microscopy. JXA-6400 device was used for the study. The view of the plate surface is shown in figure 7. Light spots correspond to the areas where the thin film broke away from the plate surface, darker areas where the film remained on the plate surface. It can be seen that the film remained on the plate surface for most of the plate surface. This means that the actual adhesion strength of the film to the metal may be slightly higher than the value obtained during measurements: σ tear ≥ 1.84 MPa.
In the next experiment to determine the adhesive strength of asmol on tear-off, similar rods were used. Since the determination of the actual adhesion is hindered by the low cohesive strength of the mastic itself, the «soft layer» effect was used. A soft layer is a thin layer of material (a layer of asmol mastic) with low rigidity and high deformability. The main effect is that the strength of the soft layer (in this case mastic between the ends of the rods) is inversely proportional to the relative thickness of the layer (thickness to diameter ratio). By reducing the thickness of the asmol layer, it is possible to increase the failure load until true breakaway adhesion is obtained.
Table 3 shows the results of these experiments. Moreover, all failures occurred by cohesive mechanism (by mastic). This means that the true adhesive strength can be greater than the highest of the obtained results: σadhes > 1.67 MPa.
After asmol is applied to steel, a thin film with a thickness of complex chemical and phase composition is formed on the steel surface. Protective properties of asmol are largely determined by the properties of this film. Therefore, the adhesion force of the formed film to steel should be taken as the true adhesion [14].
Regularities of adhesion of asmol materials in the process of its production and operation in real conditions have been studied.
The method of asmol synthesis allowed to obtain the dependence of the adhesion value on the reaction time. Complete consumption of sulfuric acid in the process of chemical reaction is accompanied by stability of adhesion value.
The results of adhesion determination of industrial samples of LIAM asphalt material confirm the stability of adhesion parameters.
Stability of adhesion indicators of asmol coating obtained after 6 years of operation, as well as cohesive nature of detachment in conjunction with the above characteristics indirectly indicate the presence of chemical adhesion of asmol with the metal surface.
The results of laboratory studies of actual adhesion exceed by an order of magnitude the values of normative documentation and confirm the hypothesis of chemical interaction of asmol with metal.
The formation of chemical adhesion of asmol coating allows to provide additional protection against corrosion and as a consequence increases the reliability and safety of main pipelines [14].
1 P.F. Koshenskov, O.V. Konoplyannikov, A. Skosyrev, V.S. Smirnov, A.V. Vavilov, Series: Natural and Technical Sciences 11 (2015) 51.
2 W.-F. Su, Principles of Polymer Design and Synthesis, Springer 82 (2013) 61. https://doi.org/10.1007/978-3-642-38730-2_4
3 M. Mohammed, T. Parry, N. Thom, J. Grenfell, Construction and Building Materials 190 (2018) 382. https://doi.org/10.1016/j.conbuildmat.2018.09.084
4 I.F. Gladkih, M.A. Gladkih, P.I. Solonin, R.N. Bahtizin, S.V. Kuznecov, V.V. Sokolova, Neftegazovoe delo – Oil and Gas Business 22(4) (2024) 304. https://doi.org/10.17122/ngdelo-2024-4-304-312
5 Patent RF, No. 2 746 727, I.F. Gladkikh, A.N. Timofeev, E.Yu. Seredyuk, R.V. Khvan Asmol Production Method and Anticorrosive Insulating Tape (2021).
6 GOST 26589-94. Roofing and waterproofing mastics. Test methods. Available at: https://docs.cntd.ru/document. (21.01.2025).
7 X. Zou, Ch. Ke, ACS Applied Materials & Interfaces 14(23) (2022) 27383. https://doi.org/10.1021/acsami.2c04971
8 N.M. Cherkasov, Corrosion of Neftegaz Territory (2014) 58.
9 I.F. Gladkikh, Yu.V. Danilenko, S.V. Pestrikov, Neftegazovoe delo 1 (2015) 97.
10 N.M. Cherkasov, I.F. Gladkikh, N.M. Zagretdinova, K.M. Gumerov, Corrosion of Neftegaz Territory 3 (2007) 24.
11 I.F. Gladkikh, T.V. Dmitrieva, D.E. Bugai, M.A. Gladkikh, Online Edition «Oil and Gas Business» 4 (2023) 17. https://doi.org/10.17122/ogbus-2023-4-17-39
12 GOST 51164-98, Steel Pipe Mains. General Requirements for Corrosion Protection. Available at: https://docs.cntd.ru/document/1200001879 (21.01.2025).
13 A.D. Batte, R.R. Fessler, J.E. Marr, S.C. Rapp, 2012 9th International Pipeline Conference, Calgary (2012) 379. https://doi.org/10.1115/IPC2012-90231
14 N.M. Cherkasov, I.F. Gladkikh, U.V. Danilenko, Theory and Practice of Corrosion Protection 4 (2015) 59.
R.N. Bakhtizin, M.A. Gladkikh, R.M. Karimov, I.F. Gladkikh, Study of adhesion properties of asmol materials in the process of their operation and production, UNEC J. Eng. Appl. Sci. 5(1) (2025) 26-34. https://doi.org/10.61640/ujeas.2025.0503
Anyone you share the following link with will be able to read this content:

This article is licensed under the Creative Commons Attribution ( CC BY 4.0 ) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
P.F. Koshenskov, O.V. Konoplyannikov, A. Skosyrev, V.S. Smirnov, A.V. Vavilov, Series: Natural and Technical Sciences 11 (2015) 51.
W.-F. Su, Principles of Polymer Design and Synthesis, Springer 82 (2013) 61. https://doi.org/10.1007/978-3-642-38730-2_4
M. Mohammed, T. Parry, N. Thom, J. Grenfell, Construction and Building Materials 190 (2018) 382. https://doi.org/10.1016/j.conbuildmat.2018.09.084
I.F. Gladkih, M.A. Gladkih, P.I. Solonin, R.N. Bahtizin, S.V. Kuznecov, V.V. Sokolova, Neftegazovoe delo – Oil and Gas Business 22(4) (2024) 304. https://doi.org/10.17122/ngdelo-2024-4-304-312
Patent RF, No. 2 746 727, I.F. Gladkikh, A.N. Timofeev, E.Yu. Seredyuk, R.V. Khvan Asmol Production Method and Anticorrosive Insulating Tape (2021).
GOST 26589-94. Roofing and waterproofing mastics. Test methods. Available at: https://docs.cntd.ru/document. (21.01.2025).
X. Zou, Ch. Ke, ACS Applied Materials & Interfaces 14(23) (2022) 27383. https://doi.org/10.1021/acsami.2c04971
N.M. Cherkasov, Corrosion of Neftegaz Territory (2014) 58.
I.F. Gladkikh, Yu.V. Danilenko, S.V. Pestrikov, Neftegazovoe delo 1 (2015) 97.
N.M. Cherkasov, I.F. Gladkikh, N.M. Zagretdinova, K.M. Gumerov, Corrosion of Neftegaz Territory 3 (2007) 24.
I.F. Gladkikh, T.V. Dmitrieva, D.E. Bugai, M.A. Gladkikh, Online Edition «Oil and Gas Business» 4 (2023) 17. https://doi.org/10.17122/ogbus-2023-4-17-39
GOST 51164-98, Steel Pipe Mains. General Requirements for Corrosion Protection. Available at: https://docs.cntd.ru/document/1200001879 (21.01.2025).
A.D. Batte, R.R. Fessler, J.E. Marr, S.C. Rapp, 2012 9th International Pipeline Conference, Calgary (2012) 379. https://doi.org/10.1115/IPC2012-90231
N.M. Cherkasov, I.F. Gladkikh, U.V. Danilenko, Theory and Practice of Corrosion Protection 4 (2015) 59.