UNEC Journal of Engineering and Applied Sciences Volume 5, No 2, pages 116-126 (2025) Cite this article, 142 https://doi.org/10.61640/ujeas.2025.1211
Organogold(I) chemistry, particularly complexes featuring isocyanide ligands, has been a field of active research for over half a century [1-4]. These compounds are of significant interest due to their diverse applications, notably as phosphorescent materials [5-8] and efficient catalysts in organic synthesis [9-13]. The Lewis acidic nature of the gold(I) center and its carbophilicity is key to its reactivity, enabling the activation of unsaturated substrates such as isocyanides, which serve as classic σ-donor ligands in coordination chemistry.
The conventional synthetic route to aryl gold(I) isocyanide complexes relies on the transmetallation from organomagnesium reagents [8,14], a method often complicated by anhydrous conditions and the sensitivity of the reagents. In contrast, transmetallation from boronic acids [11,12,15] presents a more robust and versatile alternative, a reaction step also identified as a key intermediate in gold-catalyzed Sonogashira-type couplings. This boronic acid pathway potentially facilitates access to a wider range of functionalized aryl complexes, enabling the systematic exploration of structure-property relationships.
While the molecular structures of such complexes are often linear, their solid-state supramolecular architectures are dictated by weak, yet highly influential, non-covalent interactions. Among these, aurophilic interactions [4,16,17] are a hallmark of gold(I) chemistry and are known to profoundly impact photophysical properties [18,19]. However, the predictable formation of specific oligomeric species, such as discrete tetramers, through a combination of aurophilic and other weak forces remains an area of keen interest.
In this context, we report the synthesis and detailed structural analysis of a novel benzo[b]thiophen-2-yl gold(I) tert-butyl isocyanide complex. Utilizing the practical boronic acid transmetallation route, we obtained the target complex and uncovered its solid-state structure.
The title compound was prepared according to a literature procedure15(Fig. 1). Chloro(dimethyl sulfide)gold(I) (15.0 mg, 0.051 mmol, 1.0 eq) and tert-butyl isocyanide (4.2 mg, 0.051 mmol, 1.0 eq) were dissolved in dichloromethane (2 ml) in a 10 ml vial equipped with a magnetic stir bar. Benzo[b]thiophene-2-boronic acid (9.1 mg, 0.051 mmol, 1.0 eq) was dissolved in methanol (2 ml) and added to a solution under stirring, then sodium carbonate (54.0 mg, 0.51 mmol, 10 eq) was added. After 1 hour of stirring the mixture was filtered, the solution was evaporated and the residue was purified by column chromatography (eluent: DCM/hexane, 1:1), then evaporated and dried under vacuum. White solid. Yield: 7.8 mg (37%). Crystals suitable for X-ray analysis were obtained by layering hexane over a dichloromethane solution of the target complex.
A suitable single crystal was carefully selected and mounted on a glass fiber prior to data acquisition. Diffraction data were collected using a Bruker SMART APEX II diffractometer equipped with Mo-Kα radiation (λ = 0.71073 Å) at ambient temperature. Standard corrections for Lorentz, polarization, and absorption effects were applied to the collected intensities. The crystal structure was determined employing direct methods as implemented in the SHELXT [24] program and subsequently refined using full-matrix least-squares procedures on F² within the SHELXL [25] framework. Hydrogen atoms were placed in calculated positions and refined using a riding model, with C–H distances of 0.95–0.98 Å and Uiso(H) values set to 1.5 Ueq(C) for methyl groups and 1.2 Ueq(C) for aromatic carbon atoms.
The thiophene ring (S2/C19–C22) in molecule II is rotationally disordered (flip disorder) by ca 180° (around the single C14—Au2 bond, to which it is attached) over two sites with the site-occupation factors of 0.55 and 0.45 (fixed after refinement cycles). For these rings, FLAT, SADI and EADP instructions were also used. Due to weak agreement between the measured and calculated intensities, nineteen reflections were excluded from the final refinement. (0 1 1, 0 -1 1, -15 8 2, -14 10 6, -12 9 -7, -8 2 12, -11 2 6, -1 6 16, 2 -2 -14, -7 7 16, -3 -3 -3, -3 -17 -1, -13 9 -3, -11 9 7, 1 6 -2, -2 0 -16, 4 2 -17, 0 11 11, 11 2 -8) were omitted during the final refinement cycle. ORTEP-3 [26] and Platon [29] software was used for graphics. The experimental parameters and their particulars are itemized in table 1.
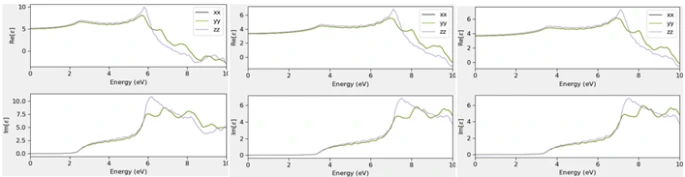
Figure 4. Calculated real and imaginary part of dielectric function of bulk bilayer and monolayer structure of GaAs
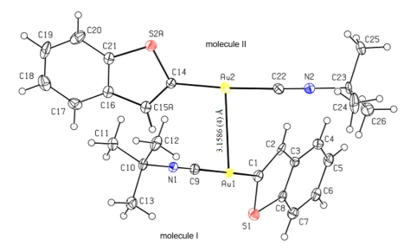
Figure 2. The molecular structure of the compound, showing the atom- numbering scheme. Displacement ellipsoids are drawn at the 30 % probability level. The minor component of the disorder has been omitted for clarity
The complex crystallizes in the monoclinic P21/n space group with Z = 8 (figure 1). Figure 2 shows an overlay plot of two molecules in the asymmetric unit, with an r.m.s. deviation of 0.725 Å. Except for the atoms of the minor part of the disordered molecule and the butyl group, all atoms of two molecules are quite compatible and coincide with each other. The asymmetric unit contains one-half of the tetrameric molecule (figure 3), the center of which coincides with an inversion center.
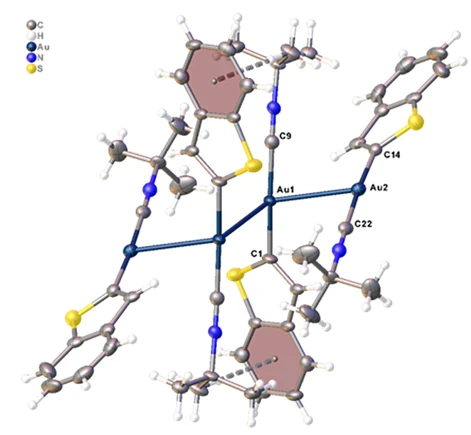
Figure 4. Molecular structure of the title compound. Displacement ellipsoids are drawn at the 50% probability level
The tetramer adopts a zigzag configuration, which is stabilized by aurophilic interactions between the gold atoms with Au···Au distances of 3.1586(4) and 3.1837(6) Å and an Au-Au-Au angle of 107.259(14)° (table 2). Each gold(I) center exhibits the typical linear coordination geometry, with C–Au–C angles of 175.6(3)° and 175.9(3)° (table 2). The Au–C bond lengths to the isocyanide and aryl carbon atoms are 1.991(8)/1.975(8) Å and 2.021(8)/2.023(8) Å, respectively (table 1). The central units of the tetramer are arranged in a strictly head-to-tail fashion, as evidenced by a C1–Au1···Au1'–C1' torsion angle of 180.0°. In contrast, the terminal fragments are oriented with a C1–Au1···Au2–C14 torsion angle of 114.2°. The stability of the tetrameric framework is further enhanced by a pair of C–H···π interactions (figure 4, table 3). The other bond lengths and angles are comparable to those in the similar structures discussed in a recently published study [25].
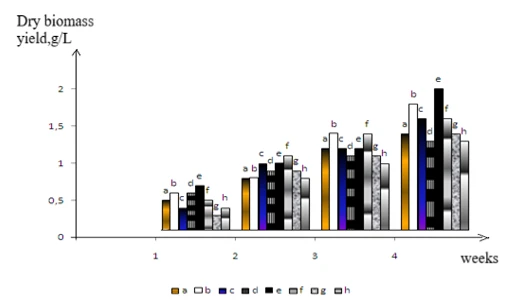
Figure 1. Biomass yield of the bacterial consortium when grown on brominated phenolic and aromatic compounds: a- ortho-bromophenol, b- para-bromophenol, c- meta-bromophenol, d- bromobenzene, e- tetrabromopyrocatechol, f- para-bromotoluene, meta-bromotoluene, ortho-bromotoluene
In the crystal lattice, the tetramers are interconnected primarily via weak C–H···S hydrogen bonds (figures 5 to 8, table 2). Additional stabilization is provided by C–H···π contacts involving the central and terminal benzothiophene fragments of adjacent molecules (figure 6, table 3).
Hirshfeld surfaces and two-dimensional fingerprint plots have been generated to visualize the intermolecular contacts of the compound using Crystal Explorer program [26]. These intercontacts are featured by normal mapping of dnorm on molecular Hirshfeld surfaces (figure 9a). The 3D Hirshfeld Surface mapped over dnorm between -0.1282 a.u (blue) and 1.3969 a.u (red). White denotes contacts with distances equal to the van der Waals (vdW) radii. Connections that are short of the vdW radii are represented in red, while those that exceed the vdW radii are shown in blue. In figure 7a, dark-red spots represent strong intermolecular C—H⋯S interactions. In addition, the shape-index is used to identify complementary hollows (red) and bumps (blue) where two molecular surfaces touch one another (figure 9b). This includes π–π stacking, represented by adjacent red and blue triangles. In this structure there are C---H… π interactions and no π–π stacking interaction. Curvedness is a tool for pinpointing planar stacking configurations and how neighbouring molecules interact. Figure 9c shows relatively large green planes in the nine-membered ring systems separated by blue edges. These green planes give us an idea of the flatness of complexes, and the fragment patch (figure 9d) is designed to indicate the nearest neighbouring molecule [7].
In the crystal structure of the compound, intermolecular C—H···S and C—H···p interactions were observed. The two-dimensional fingerprint plots show that the main contributors to crystal packing are H…H contacts (54.8 %, figure 10b), C…H/H…C (25.9 %, figure 10c), S…H/H…S (7.2 %, figure 10d) and Au…H/H…Au (6.0 %, figure 10e). The remaining small contributions are C…C (1.5 %), S…C/C…S (1.3 %), Au…C/C…Au (1.0 %), Au…Au (0.8 %), N…C/C…N (0.5 %), N…H/H…N (0.5 %), Au…N/N…Au (0.3 %), and S…N/N…S (0.2 %). The optimized molecular geometry corresponded well to the observed solid-state geometry. This supports the crystallographic findings and implies that the molecular structure is stable.
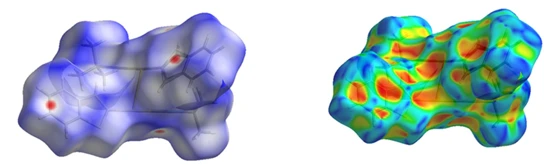
Figure 9. Hirshfeld surface mapped over dnorm for compound, showing intermolecular contacts. Hirshfeld surfaces for the compound mapped with a) dnorm b) shape index, c) curvedness and fragment patch for the compound
a) b)
In conclusion, we have successfully synthesized a novel benzo[b]thiophen-2-yl gold(I) tert-butyl isocyanide complex through a practical and robust transmetallation reaction from the corresponding boronic acid. This method offers a valuable alternative to the more sensitive organomagnesium-based routes, potentially broadening access to functionalized aryl gold(I) isocyanide complexes. Single-crystal X-ray diffraction analysis revealed that the complex does not exist as discrete monomers in the solid state but instead self-assembles into a well-defined zigzag-shaped tetramer. This supramolecular architecture is primarily stabilized by significant aurophilic interactions (Au···Au 3.1586(4) - 3.1837(6) Å), which dictate the tetrameric arrangement.
1 H. Ecken, M.M. Olmstead, B.C. Noll, S. Attar, B. Schlyer, A. L. Balch, Journal of the Chemical Society, Dalton Transactions 22 (1998) 3715. https://doi.org/10.1039/A805086D
2 G. Jia, N.C. Payne, J.J. Vittal, R.J. Puddephatt, Organometallics 12(12) (1993) 4771. https://doi.org/10.1021/om00036a017
3 R. Usón, A. Laguna, J. Vicente, J. García, B. Bergareche, P. Brun, Inorganica Chim Acta 28 (1978) 237. https://doi.org/10.1016/S0020-1693(00)87441-1
4 N.Q. Shixaliyev, A.M. Maharramov, A.V. Gurbanov, N.V. Gurbanova, V.G. Nenajdenko, V.M. Muzalevskiy, K.T. Mahmudov, M.N. Kopylovich, Journal of Molecular Structure 1041 (2013) 213. https://doi.org/10.1016/j.molstruc.2013.03.024
5 M.V. Grudova, A.S. Novikov, A.S. Kubasov, V.N. Khrustalev, A.A. Kirichuk, V.G. Nenajdenko, A.G. Tskhovrebov, Crystals (Basel) 12(5) (2022) 613. https://doi.org/10.3390/cryst12050613
6 H. Ito, T. Seki, Bull Jpn Soc Coord Chem 62 (2013) 3. https://doi.org/10.4019/bjscc.62.3
7 T. Seki, N. Tokodai, S. Omagari, T. Nakanishi, Y. Hasegawa, T. Iwasa, T. Taketsugu, H. Ito, J Am Chem Soc 139(19) (2017) 6514. https://doi.org/10.1021/jacs.7b00587
8 S. Coco, C. Cordovilla, P. Espinet, J. Martín-Álvarez, P. Muñoz, Inorg Chem 45(25) (2006) 10180. https://doi.org/10.1021/ic060702a
9 J. Wang, Z.-Y. Wang, P. Luo, B. Li, L.-Y. Wang, S.-Q. Zang, Crystal Growth & Design 19(2) (2019) 538. https://doi.org/10.1021/acs.cgd.8b01495
10 Y. Xu, X. Hu, J. Shao, G. Yang, Y. Wu, Z. Zhang, Green Chemistry 17(1) (2015) 532. https://doi.org/10.1039/C4GC01322K
11 A.R. Asgarova, A.N. Khalilov, I. Brito, A.M. Maharramov, N.G. Shikhaliyev, J. Cisterna, A. Cárdenas, A.V. Gurbanov, F.I. Zubkov, K.T. Mahmudov, Acta Crystallographica Section C Structural Chemistry 75 (2019) 342. https://doi.org/10.1107/S2053229619001025
12 K. Özkaraca, M. Akkurt, N.Q. Shikhaliyev, U.F. Askerova, G.T. Suleymanova, G.Z. Mammadova, D.M. Shadrack, Acta Crystallographica Section E Crystallographic Communications 76 (2020) 1251. https://doi.org/10.1107/S2056989020009202
13 A. Ribeiro, I. Matias, P. Zargaran, A. Hashmi, L. Martins, Materials 14 (2021) 4294. https://doi.org/10.3390/ma14154294
14 L. Zhang, W. Zhang, D. Li, R. Yang, Z. Xia, iScience 27(1) (2024) 108531. https://doi.org/10.1016/j.isci.2023.108531
15 S. Zhang, X. Ye, L. Wojtas, W. Hao, X. Shi, Green Synthesis and Catalysis 2(1) (2021) 82. https://doi.org/10.1016/j.gresc.2021.01.008
16 D.S. Bolotin, N.A. Bokach, A.S. Kritchenkov, M. Haukka, V.Yu. Kukushkin, Inorganic Chemistry 52(11) (2013) 6378. https://doi.org/10.1021/ic4000878
17 X. Deng, S. Liu, C. Fan, H. Liu, Y. Zou, H. He, D. Deng, S. Pu, Z. Chen, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 321 (2024) 124712. https://doi.org/10.1016/j.saa.2024.124712
18 K. Ozkaraca, M. Akkurt, N.Q. Shikhaliyev, U.F. Askerova, G.T. Suleymanova, I.M. Shikhaliyeva, A. Bhattarai, Acta Crystallographica Section E 76 (2020) 811. https://doi.org/10.1107/S2056989020006106
19 K.G. Pavlov, M.R. Grudova, A.S. Kubasov, V.N. Khrustalev, I.S. Kritchenkov, R.M. Gomila, A. Frontera, A.G. Tskhovrebov, CrystEngComm 27 (2025) 7021. https://doi.org/10.1039/D5CE00724K
20 A.G. Tskhovrebov, A.S. Novikov, A.S. Kritchenkov, V.N. Khrustalev, M. Haukka, Zeitschrift für Kristallographie 235(10) (2020) 477. https://doi.org/doi:10.1515/zkri-2020-0045
21 A.G. Tskhovrebov, A.S. Novikov, B.S. Tupertsev, A.A. Nazarov, A.A. Antonets, A.A. Astafiev, A.S. Kritchenkov, A.S. Kubasov, V.G. Nenajdenko, V.N. Khrustalev, Inorganica Chimica Acta 522 (2021) 120373. https://doi.org/10.1016/j.ica.2021.120373
22 R.L. White-Morris, M.M. Olmstead, A.L. Balch, Journal of the American Chemical Society 125(4) (2003) 1033. https://doi.org/10.1021/ja020902v
23 X.-Y. Zhai, L. Zhao, Nature Communications 16(1) (2025) 405. https://doi.org/10.1038/s41467-025-55842-w
24 G.M. Sheldrick, Acta Crystallographica A 71 (2015) 3. https://doi.org/10.1107/S2053273314026370
25 G.M. Sheldrick, Acta Crystallographica C 71 (2015) 3. http://dx.doi.org/10.1107/S2053229614024218
26 L.J. Farrugia, Journal of Applied Crystallography 45 (2012) 849. https://doi.org/10.1107/S0021889812029111
27 A.L. Spek, Acta Crystallographica E 76 (2020) 1.
28 Bruker, SADABS, Bruker AXS LLC, Madison, Wisconsin, USA (2016).
29 K.G. Pavlov, M.R. Grudova, A.S. Kubasov, V.N. Khrustalev, I.S. Kritchenkov, R.M. Gomila, A. Frontera, A.G. Tskhovrebov, CrystEngComm 27 (2025) 7021. https://doi.org/10.1039/D5CE00724K
30 P.R. Spackman, M.J. Turner, J.J. McKinnon, S.K. Wolff, D.J. Grimwood, D. Jayatilaka, M.A. Spackman, Journal of Applied Crystallography 54 (2021) 1006. https://doi.org/10.1107/S1600576721002910
O.V. Repina, A.I. Korolev, A.S. Kubasov, A.G. Tskhovrebov, N.Q. Shikhaliyev, A.A. Niyazova, M. Akkurt, Synthesis and crystal structure of benzo[b]thiophen-2-ylgold(I) tert-Butyl Isocyanide , UNEC J. Eng. Appl. Sci. 5(2) (2025) 116-126. https://doi.org/10.61640/ujeas.2025.1211
Anyone you share the following link with will be able to read this content:

This article is licensed under the Creative Commons Attribution ( CC BY 4.0 ) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
H. Ecken, M.M. Olmstead, B.C. Noll, S. Attar, B. Schlyer, A. L. Balch, Journal of the Chemical Society, Dalton Transactions 22 (1998) 3715. https://doi.org/10.1039/A805086D
G. Jia, N.C. Payne, J.J. Vittal, R.J. Puddephatt, Organometallics 12(12) (1993) 4771. https://doi.org/10.1021/om00036a017
R. Usón, A. Laguna, J. Vicente, J. García, B. Bergareche, P. Brun, Inorganica Chim Acta 28 (1978) 237. https://doi.org/10.1016/S0020-1693(00)87441-1
N.Q. Shixaliyev, A.M. Maharramov, A.V. Gurbanov, N.V. Gurbanova, V.G. Nenajdenko, V.M. Muzalevskiy, K.T. Mahmudov, M.N. Kopylovich, Journal of Molecular Structure 1041 (2013) 213. https://doi.org/10.1016/j.molstruc.2013.03.024
M.V. Grudova, A.S. Novikov, A.S. Kubasov, V.N. Khrustalev, A.A. Kirichuk, V.G. Nenajdenko, A.G. Tskhovrebov, Crystals (Basel) 12(5) (2022) 613. https://doi.org/10.3390/cryst12050613
H. Ito, T. Seki, Bull Jpn Soc Coord Chem 62 (2013) 3. https://doi.org/10.4019/bjscc.62.3
T. Seki, N. Tokodai, S. Omagari, T. Nakanishi, Y. Hasegawa, T. Iwasa, T. Taketsugu, H. Ito, J Am Chem Soc 139(19) (2017) 6514. https://doi.org/10.1021/jacs.7b00587
S. Coco, C. Cordovilla, P. Espinet, J. Martín-Álvarez, P. Muñoz, Inorg Chem 45(25) (2006) 10180. https://doi.org/10.1021/ic060702a
J. Wang, Z.-Y. Wang, P. Luo, B. Li, L.-Y. Wang, S.-Q. Zang, Crystal Growth & Design 19(2) (2019) 538. https://doi.org/10.1021/acs.cgd.8b01495
Y. Xu, X. Hu, J. Shao, G. Yang, Y. Wu, Z. Zhang, Green Chemistry 17(1) (2015) 532. https://doi.org/10.1039/C4GC01322K
A.R. Asgarova, A.N. Khalilov, I. Brito, A.M. Maharramov, N.G. Shikhaliyev, J. Cisterna, A. Cárdenas, A.V. Gurbanov, F.I. Zubkov, K.T. Mahmudov, Acta Crystallographica Section C Structural Chemistry 75 (2019) 342. https://doi.org/10.1107/S2053229619001025
K. Özkaraca, M. Akkurt, N.Q. Shikhaliyev, U.F. Askerova, G.T. Suleymanova, G.Z. Mammadova, D.M. Shadrack, Acta Crystallographica Section E Crystallographic Communications 76 (2020) 1251. https://doi.org/10.1107/S2056989020009202
A. Ribeiro, I. Matias, P. Zargaran, A. Hashmi, L. Martins, Materials 14 (2021) 4294. https://doi.org/10.3390/ma14154294
L. Zhang, W. Zhang, D. Li, R. Yang, Z. Xia, iScience 27(1) (2024) 108531. https://doi.org/10.1016/j.isci.2023.108531
S. Zhang, X. Ye, L. Wojtas, W. Hao, X. Shi, Green Synthesis and Catalysis 2(1) (2021) 82. https://doi.org/10.1016/j.gresc.2021.01.008
D.S. Bolotin, N.A. Bokach, A.S. Kritchenkov, M. Haukka, V.Yu. Kukushkin, Inorganic Chemistry 52(11) (2013) 6378. https://doi.org/10.1021/ic4000878
X. Deng, S. Liu, C. Fan, H. Liu, Y. Zou, H. He, D. Deng, S. Pu, Z. Chen, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 321 (2024) 124712. https://doi.org/10.1016/j.saa.2024.124712
K. Ozkaraca, M. Akkurt, N.Q. Shikhaliyev, U.F. Askerova, G.T. Suleymanova, I.M. Shikhaliyeva, A. Bhattarai, Acta Crystallographica Section E 76 (2020) 811. https://doi.org/10.1107/S2056989020006106
K.G. Pavlov, M.R. Grudova, A.S. Kubasov, V.N. Khrustalev, I.S. Kritchenkov, R.M. Gomila, A. Frontera, A.G. Tskhovrebov, CrystEngComm 27 (2025) 7021. https://doi.org/10.1039/D5CE00724K
A.G. Tskhovrebov, A.S. Novikov, A.S. Kritchenkov, V.N. Khrustalev, M. Haukka, Zeitschrift für Kristallographie 235(10) (2020) 477. https://doi.org/doi:10.1515/zkri-2020-0045
A.G. Tskhovrebov, A.S. Novikov, B.S. Tupertsev, A.A. Nazarov, A.A. Antonets, A.A. Astafiev, A.S. Kritchenkov, A.S. Kubasov, V.G. Nenajdenko, V.N. Khrustalev, Inorganica Chimica Acta 522 (2021) 120373. https://doi.org/10.1016/j.ica.2021.120373
R.L. White-Morris, M.M. Olmstead, A.L. Balch, Journal of the American Chemical Society 125(4) (2003) 1033. https://doi.org/10.1021/ja020902v
X.-Y. Zhai, L. Zhao, Nature Communications 16(1) (2025) 405. https://doi.org/10.1038/s41467-025-55842-w
G.M. Sheldrick, Acta Crystallographica A 71 (2015) 3. https://doi.org/10.1107/S2053273314026370
G.M. Sheldrick, Acta Crystallographica C 71 (2015) 3. http://dx.doi.org/10.1107/S2053229614024218
L.J. Farrugia, Journal of Applied Crystallography 45 (2012) 849. https://doi.org/10.1107/S0021889812029111
A.L. Spek, Acta Crystallographica E 76 (2020) 1.
Bruker, SADABS, Bruker AXS LLC, Madison, Wisconsin, USA (2016).
K.G. Pavlov, M.R. Grudova, A.S. Kubasov, V.N. Khrustalev, I.S. Kritchenkov, R.M. Gomila, A. Frontera, A.G. Tskhovrebov, CrystEngComm 27 (2025) 7021. https://doi.org/10.1039/D5CE00724K
P.R. Spackman, M.J. Turner, J.J. McKinnon, S.K. Wolff, D.J. Grimwood, D. Jayatilaka, M.A. Spackman, Journal of Applied Crystallography 54 (2021) 1006. https://doi.org/10.1107/S1600576721002910