UNEC Journal of Engineering and Applied Sciences Volume 5, No 1, pages 96-114 (2025) Cite this article, 796 https://doi.org/10.61640/ujeas.2025.0511
The synthesis and structural investigation of polyfunctional compounds is a highly focused and meticulously studied area within the field of fine organic synthesis. In this context, the synthesis of dihalogendiazabutadienes via the reaction of N-substituted hydrazones of benzaldehyde derivatives with polyhalomethanes (such as CCl₄ and CBr₄) in the presence of a CuCl catalyst has garnered significant attention. This process is particularly relevant due to its potential for structural exploration through X-ray crystallographic analysis and the study of factors influencing the reaction pathway (scheme 1).[1-5]
Dihalogendiazabutadienes are notable for their high reactivity, which stems from their classification as heterodienes. The chemistry of heterodienes has long been a central topic in organic synthesis, with extensive literature exploring the behavior of related systems such as azoalkenes, nitrosoalkenes, α,β-unsaturated carbonyl compounds, and imines [6]. Heterodienes represent some of the simplest yet most valuable vinyl systems due to their distinctive reactivity, electronic structure, and synthetic utility [7]. The presence of heteroatoms within the conjugated diene framework induces polarization of the π-system, thereby enhancing their reactivity and enabling diverse reaction pathways [8].
A classification of the most commonly used heterodienes in organic synthesis—such as α,β-unsaturated carbonyl compounds (enones), 1- and 2-azadienes, 1,2-diaza-1,3-butadienes (azoalkenes), nitro- and nitrosoalkenes, 2,3-diaza-1,3-butadienes, α-dicarbonyl compounds, and α-diimines—has been previously established [8]. Notably, the azoalkene-type diene fragment synthesized by our research group is included in this classification.
In the literature, heterodienes are typically involved in two main types of reactions:
(1) They act as highly reactive Michael acceptors in nucleophilic addition reactions [8,9], and
(2) They undergo [4+2] cycloaddition (Diels–Alder) reactions with electron-rich dienophiles, owing to their electron-deficient nature [10–15].
Based on the above, it is evident that dihalogendiazabutadienes hold considerable promise as Michael acceptors in fine organic synthesis. The unique structures of the halogendiazadienes synthesized by our group provide an excellent platform for investigating non-covalent interactions, which have become a significant area of contemporary research. The presence of geminal halogen atoms, a diazo group, and multiple electron-donating and electron-withdrawing substituents enables the formation of pnicogen [5], tetrel [2], and halogen bonding interactions [16,17], underscoring the importance of their synthesis.
Azo-hydrazones, in particular, are widely applied across various domains, ranging from catalysis [18], organic and inorganic synthesis [19,20], and medicinal chemistry [21] to materials science [22]. They also serve as molecular clocks [23], analytical reagents [24], ligands [25], and dyes [26]. The donor–acceptor properties of azo-hydrazones are largely governed by the nature of their functional groups [27–34]. Furthermore, upon halogenation, the formation of halogen bonds was observed, and the molecular and crystal structures, including intermolecular interactions, were thoroughly analyzed using Hirshfeld surface analysis.
2.1 Synthesis of dichlorodiazadienes based on p-toluene benzaldehyde
It is important to note that the impact of the nature of functional groups in N-substituted phenylhydrazones and their position in yhe benzene ring has always been in the focus of attention in our research. Precisely for this reason, all reaction products (whose presence in the reaction mixture was determined using TLC) were separated using column chromatography, and their structures were determined. Thus, the determination of obtaining by-products of the reaction allowed to get a more clear understanding of reaction mechanism, of the general course of the reaction [cathalysis]. Precisely because of this research we determined the formation of several by-products in a series of reactions [35]. As a result of the research, it was concluded that the nature of functional groups in aldehyde fragment substantially affects the direction and yield of the reaction.
Various dichlorodiazadienes were synthesized via the reaction of N-substituted hydrazones [36] (synthesized from p-toluenebensaldehyde) with CCl4 under the conditions of cathalytic olefination reaction, in the presence of CuCl in cathalytic quantity.
The structure of obtained compounds 1-8 was confirmed using NMR. The monocrystals of compounds 4 and 6 were grown and their molecular structures were confirmed using X-ray structural analysis. However, it was determined that during the reaction of (E)-1-(4-methylbenzylydene)-2-(4-nitrophenyl)-hydrazone with an electronacceptor nitro-group in the hydrazine fragment, (E)-1-(4-nitrophenyl)-2-(1,1,1-trichloro-2-methylpropan-2-yl)diazene and (Z)-4-methyl-N-(4-nitrophenyl)benzohydrazonoyl chloride are formed together with the main reaction product, (E)-1-(2,2-dichloro-1-(p-tolyl)vinyl)-2-(4-nitrophenyl)diazene. The monocrystal of compound 9 was grown and its structure was confirmed using X-ray analysis method (scheme 3, figure 1).
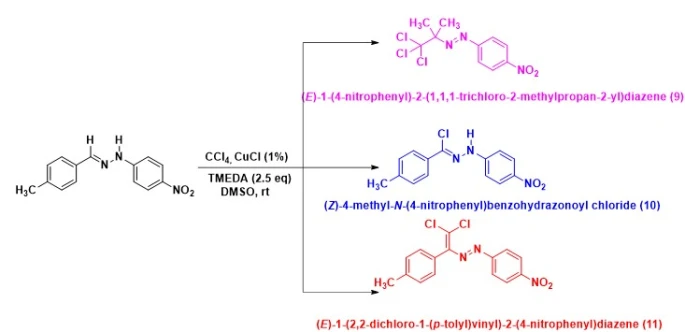
Scheme 3. The scheme of the reaction of (E)-1-(4-methylbenzylidene)-2-(4-nitrophenyl)hydrazine with CCl4
It should be noted that in the course of the reaction of (E)-1-(4-methylbenzylydene)-2-(4-nitrophenyl)hidrazone 9 with CBr4, the substitution of hydrogen atom of =CH imine for bromine occured.
The structure of (Z)-4-methyl-N(4-nitrophenyl)benzohydrazonyl was confirmed using NMR, as well as X-ray analysis method. In both reactions of (E)-1-(4-methylbenzylydene)-2-(4-nitrophenyl)-hydrazone (meaning both with CCl4 and with CBr4), the proceeding of the reaction in the new direction, with the halogenation of the hydrogen atom on the imine carbon atom, was determined. Thus, taking into account all these results, it was proven that in the course of the reaction with (E)-1-(4-methylbenzylydene)-2-(4-nitrophenyl)-hydrazone, 3 products are formed, which shows the substantial impact of nitro-group in the para-position in the hydrazine fragment on the direction of the reaction. It bears mentioning that these deviations have not been observed in other reactions
2.2 NMR interpretation
First, N-substituted phenylhydrazones were synthesized and their structures studied via NMR method. NMR spectres of hydrazones were in accordance with those reported in literature [36].
Then the corresponding dichlorodiazadienes were synthesized via reaction of phenylhydrazones with CCl4 and their structures were also interpreted using NMR method. The fact that signals corresponding to NH and CH groups are non-existent in NMR 1H spectre of dichlorodiazadienes shows the functionalization of C(sp2)-H bond in the compound. The existence of additional 1 C atom in the 150.81-162.32 range of 13C specter shows the formation of this double bond.
The fact that a singlet corresponding to 6H atom characteristic of aliphatic CH3 groups in the 1H NMR specter of (E)-1-(4-nitrophenyl)-2-(1,1,1-trichlor-2-methylpropane-2-il)diazene (compound 9) can be observed at 1.67 m.h., and also the fact that there are 2 duplets at 8.39- 8.36 and 7.91 -7.88 m.h. shows that (E)-1-(4-nitrophenyl)-2-(1,1,1-trichloro-2-methylropane-2-yl)diazene compound is obtained.
The locations and splitting of signals (7.81 m.h. (1H,s), 2.41m.h. (3H s), 8.22-8.19 (2H d), 7.62-7.60 (2H d), 7.26-7.23 (2H d), 7.16-7.13 (2H d)) in the 1H NMR specter demonstrates that the spectre belongs to (Z)-4-methyl-N(4-nitrophenyl)benzohydrazonyl chloride (compound 10).
2.3 Crystal structures
Structures of the synthesized compounds have been studied by a number of analytical methods, one of which was X-ray crystallographic method [37]. The molecular structures of compounds 4, 6, 9 and 12 are presented in Figure 1. Crystallographic data for the structural analysis have been deposited in the Cambridge Crystallographic Data Center (CCDC 2333633, 2367476, 2367477 and 2367479 for 4, for 6, or 9, for 12). Copies of this information can be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: (+44) 1223–336033. The crystallographic-structure data of the substances are presented in table 1.
Crystal structure determination
While compound (4) crystallizes in the monoclinic P21/n space group with Z = 4, compound (6) crystallizes in the monoclinic Cc space group with four independent molecules (I, II, III and IV) in the asymmetric unit (Z = 16), and compounds (9) and (12) crystallize in the monoclinic P21/c space group with Z = 4.
In compound (4) (figure 1) the angle between the two aromatic rings (C3—C8 and C10—C15) in the molecule is 89.44(6) °. The C1--N1--N2--C10, C2--C1--N1--N2 and C3--C1--N1--N2 torsion angles are 178.31(10), -170.01(11) and 12.48(16)°, respectively.
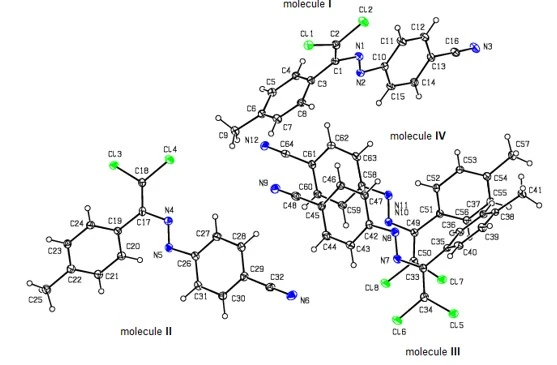
Figure 2. View of four molecules of compound (6) from the asymmetric unit with anisotropic displacement ellipsoids at the 30% probability level
The compound (6) (figure 1) has four independent molecules (I with N3, II with N6, III with N9 and IV with N12) in the asymmetric unit (figure 2). An overlay fit of inverted molecules II, III and IV on molecule I is shown in f 3, the weighted r.m.s. fit of the 21 non-H atoms being 0.903 Å and showing the major differences to be in the terminal phenyl groups (I: C10–C15, II: C26–C31, III: C42–C47 and IV: C58–C63) attached to the cyano groups of the molecules I, II, III and IV.
In the compound (6), the angles between the aromatic rings are 67.8(3)° for (I), 55.7(2)° for (II), 59.3(3)° for (III) and 47.1(2)° for (IV), respectively. The C1--N1--N2--C10, C2--C1--N1--N2 and C3--C1--N1--N2 torsion angles are 178.5(5), -174.0(5) and 9.9(8)°, respectively for (I), C17—N4—N5—C26, C18--C17—N4—N5 and C19-C17—N4—N5 torsion angles are -178.3(5), -178.7(5) and -0.9(8)°, respectively for (II), C33-N7—N8—C42, C34—C33—N7—N8 and C35—C33—N7—N8 torsion angles are 177.1(5), -175.3(5) and -9.1(8)°, respectively for (III) and C49--N10—N11—C58, C50—C49--N10—N11 and C51—C49--N10—N11 torsion angles are 179.0(5), -179.4(5) and 1.1(8)°, respectively for (IV).
In compound (9) (figure 1), the C1--N1--N2—C5, C2--C1--N1--N2, C3--C1--N1--N2 and C4--C1--N1--N2 torsion angles are 177.53(11), -133.24(12), 109.45(13) and -12.37(16)°, respectively.
In compound (12) (figure 1), the angle between the two aromatic rings (C2—C7 and C9—C14) in the molecule is 10.03(17) °. The C1--N1--N2—C9, Br1--C1--N1--N2 and C2--C1--N1--N2 torsion angles are -177.8(3), -0.7(4) and 179.5(3)°, respectively. The molecular conformation is stabilized by intramolecular N---H...Br hydrogen contacts forming the S(5) ring motif.
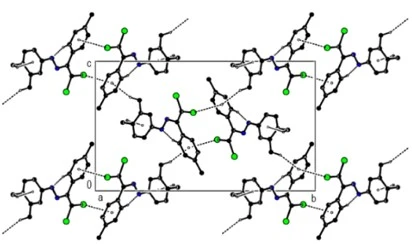
Figure 4. C---H...p and C--Cl...p interactions, and C---H...N bonds of compound (4) along the a-axis
The bond length and angle values for compound (4), (6), (9) and (12) are comparable with each other and with those of compounds reported in the literature [37-39].
In the crystal of compound (4), molecules are linked via intermolecular C---H...N hydrogen bonds, C---H..p and C---Cl...p [C2---Cl1…Cg1a : C2---Cl1 = 1.7145 (12) Å, Cl1…Cg1a = 3.4070(7), C2 …Cg1a = 5.0174(12), C2---Cl1…Cg1a = 155.47(4)°; Symmetry code (a) - x, 1 - y, 1 – z; where Cg1 is a centroid of the C3—C8 benzene ring] interactions, forming a three-dimensional network and ensuring the stability of molecular packaging (table 1, figures 4 and 5).
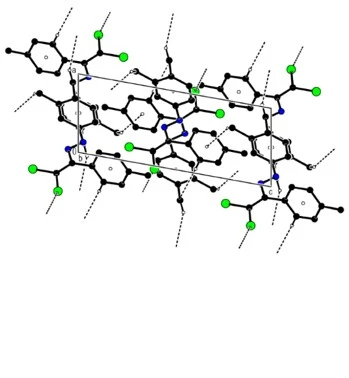
Figure 5. C---H...p and C--Cl...p interactions, and C---H...N bonds of compound (4) along the b-axis
Compound (6) crystallizes in the monoclinic Cc space group with four symmetry-independent molecules in the asymmetric unit (Z = 16). The molecules are linked in layers parallel to the (200) plane by intermolecular C---H...N hydrogen bonds and C---H...p interactions (table 1, figures 6 and 7). These layers strengthen molecular packing by bonding with van der Waals interactions.
Compound (9) crystallizes in the monoclinic P21/c space group with Z = 4. The molecules are connected in layers parallel to the (10-2) plane by intermolecular C---H...O hydrogen bonds, C---Cl... p and p-p interactions (Cg1…Cg1a = 3.6269(8) Å; symmetry code (a) -x, 1-y, 1-z; where Cg1 is a centroid of the C5—C10 benzene ring) (table 1, figures 8 and 9). These layers bond with van der Waals interactions, strengthening the crystal structure.
Compound (12) crystallizes in the monoclinic P21/c space group with Z = 4. The molecules are connected in layers parallel to the (020) plane by intermolecular C---H...O and N---H...O hydrogen bonds and C--O...p interactions (table 2, figures 10, 11 and 12). These layers bond with van der Waals interactions, strengthening the crystal structure.
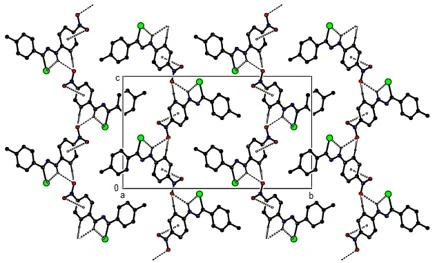
Figure 10. C---O... p, C---H...O and N---H...O interactions plus intra H-bonds of compound (12) along the a-axis
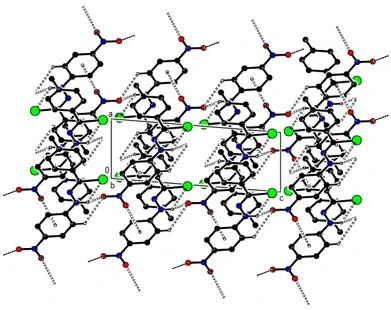
Figure 11. C---O... p, C---H...O and N---H...O interactions plus intra H-bonds along of compound (12) the b-axis
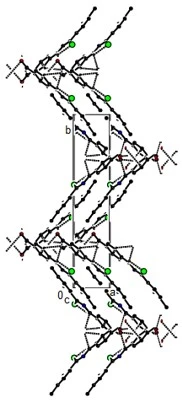
Figure 12. C---O...p, C---H...O and N---H...O interactions plus intra H-bonds along of compound (12) the c-axis
2.4. Hirshfeld surface analysis of (e)-1-(2,2-dıchloro-1-(p-tolyl)vınyl)-2-(3,4-dımethylphenyl)diazene (compound 4).
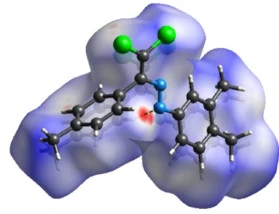
Figure 14. View of the three-dimensional Hirshfeld surface of the compound (4). plotted over electrostatic potential energy using the STO-3 G basis set at the Hartree–Fock level of theory. Hydrogen-bond donors and acceptors are shown as blue and red regions around the atoms corresponding to positive and negative potentials, respectively
In order to visualize the intermolecular interactions in the crystal of the compound (4), a Hirshfeld surface analysis was carried out using Crystal Explorer (Version 17.5) [40]. In the Hirshfeld surface plotted over dnorm (figure 13), the white surface indicates contacts with distances equal to the sum of the van der Waals radii, and the red and blue colours indicate distances shorter or longer than the van der Waals radii, respectively [41]. The bright-red spots indicate their roles as the respective donors and/or acceptors; they also appear as blue and red regions corresponding to positive and negative potentials on the Hirshfeld surface mapped over electrostatic potential, as shown in figure 14. The blue regions indicate the positive electrostatic potential (hydrogen-bond donors), while the red regions indicate the negative electrostatic potential (hydrogen-bond acceptors).
The overall two-dimensional fingerprint plot, figure 15a, and those delineated into H⋯H, H⋯Cl/Cl⋯H and H⋯C/C⋯H are illustrated in figures 15b–d, respectively, together with their relative contributions to the Hirshfeld surface. The most important interaction is H⋯H, contributing 43.1% to the overall crystal packing, which is reflected in figure 15b as the widely scattered points of high density due to the large hydrogen content of the molecule with the tips at de + di = 2.30 Å.
The symmetrical pair of spikes resulting in the fingerprint plot delineated into H⋯Cl/Cl⋯H contacts [figure 15(c)] has a 23.0 % contribution to the HS with the tips at de + di = 2.80 Å. The H⋯C/C⋯H contacts, contributing 19.8 % to the overall crystal packing, are reflected in figure 15(d) with the tips at de + di = 2.60 Å. Finally, the H⋯N/N⋯H (5.3 %), C⋯Cl/Cl⋯C (3.6 %), C⋯N/N⋯C (2.4%), Cl⋯N/N⋯Cl (1.4%), C⋯C (0.7%) and Cl⋯Cl (0.6%) contacts to the Hirshfeld surface have very low distributions of points.The large number of H⋯H, H⋯Cl/Cl⋯H and H⋯C/C⋯H interactions suggest that van der Waals interactions and hydrogen bonding play the major roles in the crystal packing [41].
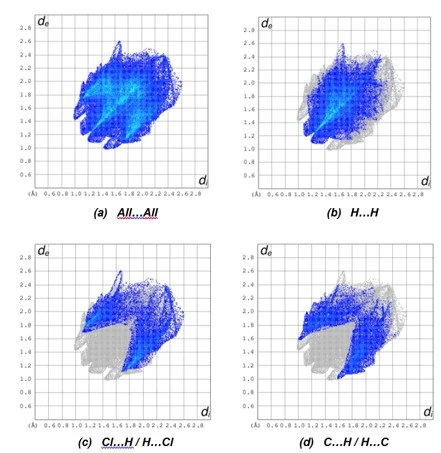
Figure 15. The full two-dimensional fingerprint plots for the compound (4), showing (a) all interactions, and delineated into (b) H…H, (c) Cl…H / H…Cl and (d) C…H / H…C interactions. The di and de values are the closest internal and external distances (in Å) from given points on the Hirshfeld surface contacts
Experimental part
2.5 General remarks
The syntheses of compounds were carried out at the Chemistry Department of Baku State University (Azerbaijan). Unless stated otherwise, all the reagents used in this study were obtained from the commercial sources (Aldrich, TCI-Europe, Strem, ABCR). The Hirshfeld surface analysis of compound (4) has been performed by using the Crystal Explorer program (version 17.5, Perth, Australia) [40]. The normalized contact distances (dnorm) based on Bondi’s van der Waals radii [41] were mapped into the Hirshfeld surfaces. SiMe4 (TMS) was used as an internal standard. Thin-layer chromatography (TLC) was performed on silhouette plate UB-254 and acidified KMnO4 solution; UV lamp rays were used to make spots visible. Column chromatography was performed on silica gel of Merk firm (63-200). All compounds were crystallized from a methylene chloride and hexane (1:3, 1:5) solvent system.
2.6 NMR anlaysis
The NMR experiments have been performed on a BRUKER FT NMR spectrometer AVANCE 300 (Bruker, Karlsruhe, Germany) (300 MHz for 1H and 75 MHz for 13C) with a BVT 3200 variable temperature unit in 5 mm sample tubes using Bruker standard software (TopSpin 3.1). Chemical shifts were given in ppm (δ) and were referenced to internal tetramethylsilane (TMS). Multiplicities are declared as follow: s (singlet), d (doublet), t (triplet), q (quadruplet), m (multiplet). Coupling constants J are given in Hz. The experimental parameters for 1H are as follows: digital resolution = 0.23 Hz, SWH = 7530 Hz, TD = 32 K, SI = 16 K, 90° pulse-length = 10 ms, PL1 = 3 dB, ns = 1, ds = 0, d1 = 1 s and for 13C as follows: digital resolution = 0.27 Hz, SWH = 17 985 Hz, TD = 64 K, SI = 32 K, 90° pulse length = 9 ms, PL1 = 1.5 dB, ns = 100, ds = 2, d1 = 3 s. The NMR-grade DMSO-d6 (99.7%, containing 0.3% H2O), CDCl3, CD3OD was used for the solutions of synthesized compound
985 Hz, TD = 64 K, SI = 32 K, 90° pulse length = 9 ms, PL1 = 1.5 dB, ns = 100, ds = 2, d1 = 3 s. The NMR-grade DMSO-d6 (99.7%, containing 0.3% H2O), CDCl3, CD3OD was used for the solutions of synthesized compound
2.7 X-RAY anlaysis
The data collection for single-crystal structure analysis was performed using an XtaLAB Synergy, Dualflex, HyPix diffractometer from Rigaku with Cu Kα radiation (l = 1.54184 Å) at 100 K. The crystallographic data are summarized in table 2. Data were corrected for Lorentz, polarisation and absorption factors. The structure was solved by direct methods and refined using SHELXT and SHELXL [36]. All H atoms were geometrically fixed and allowed to ride on their parent C atoms with C---H = 0.95 - 0.98 Å, and Uiso(H) = 1.5 Ueq(C) of the attached C atoms for methyl H atoms and 1.2 Ueq for aromatic H atoms.
2.8 General procedure for synthesis of dichlorodiazadienes
Compounds were synthesized according to the reported method [1-5]. A 20-mL screw neck vial was charged with DMSO (10 mL), obtained hydrazones (1mmol) [36], respectively, tetramethylethylenediamine (TMEDA) (295 mg, 2.5mmol), CuCl (2 mg, 0.02 mmol) and CCl4 (20 mmol, 10 equiv). After 1-3 hours (until TLC analysis showed complete consumption of the corresponding Schiff base), the reaction mixture was poured into an ~0.01 M solution of HCl (100 mL, ~pH 2- 3), and extracted with dichloromethane (3x20 mL). The combined organic phase was washed with water (3x50mL), followed by brine (30 mL), dried over anhydrous Na2SO4 and concentrated in vacuo by rotary evaporator. The residue was purified by column chromatography on silica gel using appropriate mixtures of hexane and dichloromethane (3/1-1/1), and the corresponding diazenes were obtained. Single crystal of the substances was prepared from a mixture of n-hexaneand CH2Cl2 solvents by slow evaporation.
Compound 1. (E)-1-(2,2-dichloro-1-(p-tolyl)vinyl)-2-phenyldiazene
The title compound was prepared according to the general procedure using (E)-1-(4-methylbenzylidene)-2-phenylhydrazine to afford the title compound an orange solid. Yield 51 %. M.p. 99⁰C. Anal. Calcd for C15H12Cl2N2 (M=290,17).1H NMR (300 MHz, Chloroform-d) δ 7.80 (d, J = 8.8 Hz, 2H, arom), 7.50 – 7.42 (m, 3H, arom), 7.26 (s, 2H, arom), 7.11 (d, J = 7.9 Hz, 2H, arom), 2.43 (s, 3H, CH3), 13C NMR (75 MHz, CDCl3) δ 162.3 (CAr), 152.9 (C), 152.2 (C), 138.6 (CAr), 135.2 (CAr), 131.5 (CAr), 129.8 (CArH), 129.0 (CArH), 128.9 (CArH), 123.2 (CArH), 21.5 (CH3).
Compound 2. (E)-1-(2,2-dichloro-1-(p-tolyl)vinyl)-2-(m-tolyl)diazene.
The title compound was prepared according to the general procedure using (E)-1-(4-methylbenzylidene)-2-(m-tolyl)hydrazine to afford the title compound a red solid. Yield 69 %. M.p. 87⁰C. Anal. Calcd for C16H14Cl2N2 (M=305,20), 1H NMR (300 MHz, Chloroform-d) δ 7.75 (s, 2H, arom), 7.44 (t, J = 7.9 Hz, 1H, arom), 7.36 (d, J = 7.7 Hz, 3H, arom), 7.22 (d, J = 8.0 Hz, 2H, arom), 2.51 (s, 6H, CH3). 13C NMR (75 MHz, CDCl3) δ 153.1 (C), 152.4 (C), 138.9 (CAr), 138.6 (CAr), 134.9 (CAr), 132.4 (CAr), 130.0 (CArH), 129.7 (CAr), 129.0 (CArH), 128.9 (CAr), 124.1 (CArH), 120.3 (CArH), 21.6 (CH3), 21.4 (CH3).
Compound 3. (E)-1-(2,2-dichloro-1-(p-tolyl)vinyl)-2-(p-tolyl)diazene
The title compound was prepared according to the general procedure using (E)-1-(4-methylbenzylidene)-2-(p-tolyl)hydrazine to afford the title compound a red solid. Yield 75 %. M.p. 74⁰C. Anal. Calcd for C16H14Cl2N2 (M=305,20), 1H NMR (300 MHz, Chloroform-d) δ 7.70 (d, J = 8.2 Hz, 2H, arom), 7.31 – 7.22 (m, 4H, arom), 7.10 (d, J = 7.9 Hz, 2H, arom), 2.42 (d, J = 3.1 Hz, 6H, (CH3).). 13C NMR (75 MHz, CDCl3) δ 151.1 (C), 142.2(C), 138.5 (CAr), 130.2 (CAr), 129.9 (CArH), 129.6 (CArH), 129.2(CAr), 128.8 (CArH),, 128.2 (CAr), 123.2 (CArH),, 21.4 (CH3).
Compound 4. (E)-1-(2,2-dichloro-1-(p-tolyl)vinyl)-2-(3,4-dimethylphenyl)diazene
The title compound was prepared according to the general procedure using (E)-1-(3,4-dimethylphenyl)-2-(4-methylbenzylidene)hydrazine to afford the title compound a orange solid. Yield 51 %. M.p. 73⁰C. Anal. Calcd for C17H16Cl2N2 (M=319,22). 1H NMR (300 MHz, Chloroform-d, ppm) δ 7.55 (d, J = 14.2 Hz, 2H, arom), 7.26 – 7.15 (m, 3H, arom), 7.09 (d, J = 7.7 Hz, 2H, arom), 2.42 (s, 3H, CH3), 2.31 (s, 6H, 3,4-(CH3)2). 13C NMR (75 MHz, CDCl3 ,ppm) δ 162.3 (C), 162.1 (C) 140.9 (CAr), 137.3 (CAr), 134.5 (CAr), 130.1 (CArH), 129.8 (CArH), 129.6 (CAr), 128.8 (CArH), 124.5 (CArH), 121.3 (CAr), 120.6 (CArH), 21.4 (CH3) , 19.9 (3,4-(CH3)2), 19.7(3,4-(CH3)2). CCDC reference 2268431.
Compound 5. (E)-1-(2,2-dichloro-1-(p-tolyl)vinyl)-2-(4-metoxyphenyl)diazene
The title compound was prepared according to the general procedure using (E)-1-(4-methoxyphenyl)-2-(4-methylbenzylidene)hydrazine to afford the title compound a red solid. Yield 63 %. M.p. 104⁰C. Anal. Calcd for C16H14Cl2N2O (M=321,20). 1H NMR (300 MHz, Chloroform-d, ppm) δ 7.79 (d, J = 9.0 Hz, 2H, arom), 7.26 (d, J = 8.0 Hz, 2H,arom), 7.10 (d, J = 8.0 Hz, 2H, arom), 6.95 (d, J = 9.0 Hz, 2H, arom), 3.88 (s, 3H, OCH3), 2.42 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3, ppm) δ 155.9 (C), 155.0 (C), 147.4 (CAr), 138.4 (CAr), 138.1 (CAr) 129.9 (CArH), 129.7 (CAr), 128.8 (CArH), 125.2 (CArH), 114.1 (CArH), 55.5 (OCH3), 21.4 (CH3). CCDC reference 1984582.
Compound 6. (E)-4-((2,2-dichloro-1-(p-tolyl)vinyl)diazenyl) benzonitrile
The title compound was prepared according to the general procedure using (E)-4-(2-(4-methylbenzylidene)hydrazineyl)benzonitrile to afford the title compound a red solid. Yield 69 %. M.p. 87⁰C. Anal. Calcd for C16H11Cl2N3(M= 316,19). 1H NMR (300 MHz, Chloroform-d) δ 7.86 (d, J = 8.5 Hz, 2H, arom), 7.75 (d, J = 8.5 Hz, 2H, arom), 7.27 (d, J = 6.6 Hz, 2H, arom), 7.08 (d, J = 8.0 Hz, 2H, arom), 2.43 (s, 3H, -CH3). 13C NMR (75 MHz, CDCl3) δ 162.3 (CAr), 158.2 (CAr), 154.7 (CAr), 139.0 (CAr), 133.1 (CArH), 129.7 (CArH), 129.0 (CArH), 128.7(CAr), 123.6 (CArH), 118.4 (CAr), 114.2 (CAr), 21.5 (-CH3).
Compound 7. (E)-1-(4-chlorophenyl)-2-(2,2-dichloro-1-(p-tolyl)vinyl)diazene
The title compound was prepared according to the general procedure using (E)-1-(4-chlorophenyl)-2-(4-methylbenzylidene)hydrazine to afford the title compound a red solid. Yield 69 %. M.p. 87⁰C. Anal. Calcd for C15H11Cl3N2 (M= 325,62), 1H NMR (300 MHz, Chloroform-d, ppm) δ 7.76 (d, J = 8.5 Hz, 2H, arom), 7.44 (d, J = 8.5 Hz, 2H, arom), 7.28 (d, J = 7.7 Hz, 2H, arom), 7.11 (d, J = 7.8 Hz, 2H, arom), 2.45 (s, 3H, -CH3). 13C NMR (75 MHz, CDCl3 , ppm) δ 152.3(C), 151.3(C), 138.7 (CAr), 137.4 (CAr), 129.8 (CArH), 129.3 (CArH), 129.0 (CArH), 128.6 (CAr), 128.3 (CAr), 124.4 (CArH), 123.8 (CAr), 21.5 (-CH3).
Compound 8. (E)-1-(4-bromophenyl)-2-(2,2-dichloro-1-(p-tolyl)vinyl)diazene
The title compound was prepared according to the general procedure using (E)-1-(4-bromophenyl)-2-(4-methylbenzylidene)hydrazine to afford the title compound a red solid. Yield 56 %. M.p. 112⁰C. Anal. Calcd for C16H14Cl2N2 (M=305,20), 1H NMR (300 MHz, Chloroform-d) δ 7.72 – 7.55 (m, 1H, arom), 7.46 (d, J = 8.0 Hz, 3H, arom), 7.20 (d, J = 8.0 Hz, 4H, arom), 2.38 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3) δ 132.2 (CArH), 129.8 (CArH), 129.1 (CArH), 128.9 (CAr), 128.5 (CAr), 128.0 (CAr), 126.8 (C), 126.4 (C), 125.4 (CAr), 124.6 (CArH), 29.7(CH3).
Compound 9. (E)-1-(4-nitrophenyl)-2-(1,1,1-trichloro-2-methylpropan-2-yl)diazene
The title compound was prepared according to the general procedure using (E)-1-(4-methylbenzylidene)-2-(4-nitrophenyl)hydrazine to afford the title compound a red solid. Yield 22 %. M.p.63⁰C. Anal. Calcd for C10H10Cl3N3O2 (M=310,55). 1H NMR (300 MHz, Chloroform-d) δ 8.38 (d, J = 9.0 Hz, 2H , arom), 7.89 (d, J = 9.0 Hz, 2H, arom), 1.67 (s, 6H, CH3). 13C NMR (75 MHz, CDCl3) δ 162.3 (CAr), 134.0(CAr), 124.7 (CArH), 123.3 (CArH), 82.8 (C(CH3)2, 52.2 (CCl3), 22.2(CH3)2.
Compound 10. (Z)-4-methyl-N-(4-nitrophenyl)benzohydrazonoyl chloride
The title compound was prepared according to the general procedure using (E)-1-(4-methylbenzylidene)-2-(4-nitrophenyl)hydrazine to afford the title compound a red solid. Yield 25 %. M.p. 182⁰C. Anal. Calcd for C14H12ClN3O2 (M=336,17). 1H NMR (300 MHz, Chloroform-d) δ 8.21 (d, J = 9.2 Hz, 2H, arom), 7.81 (s, 1H, -NH), 7.61 (d, J = 8.1 Hz, 2H. arom), 7.24 (d, J = 7.9 Hz, 2H, arom), 7.14 (d, J = 9.1 Hz, 2H, arom), 2.41 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3) δ 162.3 (CCl), 150.3(CAr), 139.8(CAr), 131.9(CAr), 129.5(CArH), 126.7(CArH), 126.2(CArH), 111.6 (CArH), 110.4, (CAr), 21.5. (-CH3).
Compound 11. (E)-1-(2,2-dichloro-1-(p-tolyl)vinyl)-2-(4-nitrophenyl) diazene
The title compound was prepared according to the general procedure using (E)-1-(4-methylbenzylidene)-2-(4-nitrophenyl)hydrazine to afford the title compound a red solid. Yield 45 %. M.p. 67⁰C. Anal. Calcd for C15H11Cl2N3O2 (M=336,17). 1H NMR (300 MHz, Chloroform-d) δ 8.32 (d, J = 9.0 Hz, 2H, arom), 7.90 (d, J = 9.0 Hz, 2H, arom), 7.28 (d, J = 7.7 Hz, 2H, arom), 7.08 (d, J = 7.9 Hz, 2H, arom), 2.44 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3) δ 165.9 (CAr), 162.3 (CAr), 151.8 (CAr), 149.1 (CAr), 144.0 (CAr), 129.7 (CArH), 129.1 (CArH), 127.8 (CAr), 124.6 (CArH), 123.7 (CArH), 116.3 (CAr), 29.7 (-CH3).
Compound 12. (Z)-4-methyl-N-(4-nitrophenyl)benzohydrazonoylbromide
The title compound was prepared according to the general procedure using (E)-1-(4-methylbenzylidene)-2-(4-nitrophenyl)hydrazine to afford the title compound a red solid. Yield 56 %. M.p. 176⁰C. Anal. Calcd for C14H12ClN3O2 (M=336,17). 1H NMR (300 MHz, Chloroform-d) δ 8.41 (s, 1H, -NH), 8.23 (d, J = 7.4 Hz, 2H, arom), 7.92 – 7.73 (m, 2H, arom), 7.27 – 7.21 (m, 3H, arom), 2.43 (s, 3H, CH3). 13C NMR (75 MHz, CDCl3) δ 129.7 (CAr), 129.3 (CArH), 128.0 (CArH), 126.8 (CArH), 126.1 (CArH), 124.6 (C), 123.4 (C) 114.5 (CAr), 112.8 (CAr), 112.6 (CAr), 21.3 (-CH3).
The new dichlorodiazadienes were synthesized and we were first to determine that reaction proceeds along the new direction, with the halogenation of the hydrogen atom on the imine carbon atom in both reactions of (E)-1-(4-methylbenzylydene)-2-(4-nitrophenyl)-hydrazone with CCl4 and CBr4. In the crystal of compound (4), while molecules are linked into infinite three-dimensional network by C−H···N of hydrogen bonds, C---H…p and C---Cl…p interaction. In the crystal of the compound (6), molecules are linked in layers parallel to the (200) plane by intermolecular C---H...N hydrogen bonds and C---H...p interactions, in the crystal of the compound (9), the molecules are connected in layers parallel to the (10-2) plane by intermolecular C---H...O hydrogen bonds, C---Cl... p and p-p interactions, and in the crystal of the compound (12), the molecules are connected in layers parallel to the (020) plane by intermolecular C---H...O and N---H...O hydrogen bonds and C--O... p interactions. These layers bond with van der Waals interactions, strengthening the crystal structure. In addition, Hirshfeld surface analysis of compound (4) also revealed that the dominant interaction in the crystal structure is van der Waals interactions.
1 N.Q. Shikhaliyev, M.L. Kuznetsov, A.M. Maharramov, A.V. Gurbanov, N.E. Ahmadova, V.G. Nenajdenko, A.J. Pombeiro, CrystEngComm 21(34) (2019) 5032. https://doi.org/10.1039/C9CE00956F
2 N.Q. Shikhaliyev, N.E. Ahmadova, A.V. Gurbanov, A.M. Maharramov, G.Z. Mammadova, V.G. Nenajdenko, A.J. Pombeiro, Dyes and Pigments 150 (2018) 377. https://doi.org/10.1016/j.dyepig.2017.12.033
3 N.G. Shikhaliyev, A.M. Maharramov, K.N. Bagirova, G.T. Suleymanova, B.D. Tsyrenova, V.G. Nenajdenko, A.G. Tskhovrebov, Mendeleev Communications 31(2) (2021) 191. https://doi.org/10.1016/j.mencom.2021.03.015
4 V.G. Nenajdenko, N.G. Shikhaliyev, A.M. Maharramov, K.N. Bagirova, G.T. Suleymanova, A.S. Novikov, A.G. Tskhovrebov, Molecules 25(21) (2020) 5013. https://doi.org/10.3390/molecules25215013
5 A.M. Maharramov, N.Q. Shikhaliyev, G.T. Suleymanova, A.V. Gurbanov, G.V. Babayeva, G.Z. Mammadova, A.J. Pombeiro, Dyes and Pigments 159 (2018) 135. https://doi.org/10.1016/j.dyepig.2018.06.022
6 A.Y. Sukhorukov, Frontiers in Chemistry 12 (2024) 1403024. https://doi.org/10.3389/fchem.2024.1403024
7 C. Curti, L. Battistini, A. Sartori, F. Zanardi, Chem. Rev. 120(5) (2020) 2448. https://doi.org/10.1021/acs.chemrev.9b00481
8 S.M.M. Lopes, A.L. Cardoso, A. Lemos, T.M.V.D. Pinho E. Melo, Chem. Rev. 118(23) (2018) 11324. https://doi.org/10.1021/acs.chemrev.8b00375
9 S.M. Weinreb, Synlett 30(16) (2019) 1855. https://doi.org/10.1055/s-0037-1611899
10 S.E. Denmark, S.T. Nguyen, R.Y. Baiazitov, Heterocycles 76(1) (2008) 143. https://doi.org/10.3987/com-08- s(n)15
11 Z.M. Png, H. Zeng, Q. Ye, J. Xu, Chem. Asian J. 12 (2017) 2142. http://dx.doi.org/10.3987/COM-08-S(N)21
12 R.Y. Baiazitov, S.E. Denmark, In Methods and applications of cycloaddition reactions in organic syntheses (2013) 471. https://doi.org/10.1002/9781118778173.ch16
13 K. Selvaraj, S. Chauhan, K. Sandeep, K.C.K. Swamy, Chem. Asian J. 15(16) (2020) 2380. https://doi.org/10.1002/asia.202000545
14 P.Y. Ushakov, S.L. Ioffe, A.Y. Sukhorukov, Organic Chemistry Frontiers 9(19) (2022) 5358. https://doi.org/10.1039/D2QO00698G
15 Y. Wang, Z. Jin, L. Zhou, X. Lv, Organic and Biomolecular Chemistry 22(2) (2024) 252. https://doi.org/10.1039/D3OB01626A
16 N.G. Shikhaliyev, A.M. Maharramov, G.T. Suleymanova, G.V. Babayeva, G.Z. Mammadova, I.M. Shikhaliyeva, V.G. Nenajdenko, Organic Chemistry (2021) 67. https://doi.org/10.3390/molecules27165110
17 K. Özkaraca, M. Akkurt, N.Q. Shikhaliyev, G.Z. Mammadova, D.M. Shadrack, Acta Crystallographica Section E: Crystallographic Communications 76(8) (2020) 1251. https://doi.org/10.1107/S2056989020009202
18 K. Özkaraca, M. Akkurt, N.Q. Shikhaliyev, I.M. Shikhaliyeva, A. Bhattarai, Acta Crystallographica Section E: Crystallographic Communications 76(6) (2020) 811. https://doi.org/10.1107/S2056989020006106
19 S. Balgotra, P.K. Verma, R.A. Vishwakarma, S.D. Sawant, Catalysis Reviews 62(3) (2020) 406. https://doi.org/10.1080/01614940.2019.1702191
20 J.A. Camarero, B.J. Hackel, J.J. De Yoreo, A.R. Mitchell, The Journal of organic chemistry 69(12) (2004) 4145. https://doi.org/10.1021/jo040140h
21 W. Kaim, Angewandte Chemie International Edition in English 22(3) (1983) 171. https://doi.org/10.1002/anie.198301713
22 S.H. Rohane, A.G. Makwana, Asian Journal of Research in Chemistry 10(4) (2017) 417. https://doi.org/10.5958/0974-4150.2017.00070.0
23 J. Buckingham, Quarterly Reviews, Chemical Society 23(1) (1969) 37. https://doi.org/10.1039/QR9692300037
24 T.A. Khattab, Materials Chemistry and Physics 254 (2020) 123456 https://doi.org/10.1016/j.matchemphys.2020.123456
25 O. Tšubrik, U. Mäeorg, R. Sillard, U. Ragnarsson, Tetrahedron 60(38) (2004) 8363. https://doi.org/10.1016/j.tet.2004.07.020
26 A. Hosseinian, R. Mohammadi, S. Ahmadi, A. Monfared, Z. Rahmani, RSC advances 8(59) (2018) 33828. https://doi.org/10.1039/C8RA06423G
27 S. Crespi, N.A. Simeth, B. König, Nature Reviews Chemistry 3(3) (2019) 133. https://doi.org/10.1038/s41570-019-0074-6
28 N.Q. Shikhaliyev, K. Özkaraca, M. Akkurt, X.N. Bagirova, G.T. Suleymanova, M.S. Abdulov, S. Mlowe, Acta Crystallographica Section E: Crystallographic Communications 77(11) (2021) 1158. https://doi.org/10.1107/S2056989021010756
29 Z. Atioğlu, M. Akkurt, N.Q. Shikhaliyev, G.T. Suleymanova, K.N. Bagirova, F.A. Toze, Acta Crystallographica Section E: Crystallographic Communications 75(2) (2019) 237. https://doi.org/10.1107/S2056989019000707
30 S.T. Çelikesir, M. Akkurt, N.Q. Shikhaliyev, N.A. Mammadova, G.T. Suleymanova, V.N. Khrustalev, A. Bhattarai, Acta Crystallographica Section E: Crystallographic Communications 78(4) (2022) 404. https://doi.org/10.1107/S205698902200278X
31 Z. Atioğlu, M. Akkurt, N.Q. Shikhaliyev, N.A. Mammadova, G.V. Babayeva, V.N. Khrustalev, A. Bhattarai, Acta Crystallographica Section E: Crystallographic Communications 78(8) (2022) 804. https://doi.org/10.1107/S2056989022007113
32 N.G. Shikhaliyev, G.T. Suleymanova, A.A. Israyilova, K.G. Ganbarov, G.V. Babayeva, K.A. Garazadeh, V.G. Nenajdenko, Organic Chemistry vi (2019) 0.
33 N.G. Shikhaliyev, U.F. Askerova, S.H. Mukhtarova, A.A. Niyazova, P.V. Dorovatovskii, V.N. Khrustalev, V.G. Nenajdenko, Russian Journal of Organic Chemistry 56 (2020) 185. https://doi.org/10.1134/S1070428020020013
34 N.G. Shikhaliev, G.T. Suleymanova, K.N. Bagirova, U.F. Asgerova, K.A. Garazadeh, G.V. Babayeva, N.E. Ahmedova, V.G. Nenajdenko, Journal of Low Dimensional Systems 2(2) (2018) 24.
35 G.M. Sheldrick, Acta Crystallographica Section A: Foundations and Advances 71(1) (2015) 3. https://doi.org/10.1107/S2053273314026370
36 K. Özkaraca, M. Akkurt, N.Q. Shikhaliyev, U.F. Askerova, G.T. Suleymanova, G.Z. Mammadova, D.M. Shadrack, Acta Crystallographica Section E: Crystallographic Communications 76(8) (2020) 1251. https://doi.org/10.1107/S2056989020009202
37 N.Q. Shikhaliyev, S.A. İbrahimova, G.T. Atakishiyeva, N.E. Ahmedova, G.V. Babayeva, V.N. Khrustalev, Z. Atioğlu, M. Akkurt, A. Bhattarai, Acta Crystallographica Section E: Crystallographic Communications 80(2) (2024) 184. https://doi.org/10.1107/S2056989024000732
38 P.R. Spackman, M.J. Turner, J.J. McKinnon, S.K. Wolff, D.J. Grimwood, D. Jayatilaka, M.A. Spackman, Journal of Applied Crystallography 54(3) (2021) 1006. https://doi.org/10.1107/S1600576721002910
39 V.R. Hathwar, M. Sist, M.R. Jørgensen, A.H. Mamakhel, X. Wang, C.M. Hoffmann, K. Sugimoto, B. Iversen, IUCrJ 2(5) (2015) 563. https://doi.org/10.1107/S2052252515012130
40 G.M. Sheldrick, Acta Crystallographica Section C: Structural Chemistry 71(1) (2015) 3. https://doi.org/10.1107/S2053229614024218
41 Rigaku OD CrysAlis PRO 1.171.43.92a. Rigaku Oxford Diffraction, Yarnton, England (2024).
N.Q. Shikhaliyev, A.M. Qajar, G.T. Atakishiyeva, G.V Babayeva, A.A. Niyazova, S.H. Mukhtarova, V.N. Krustalev, M. Akkurt, A.M. Maharramov, The structural properties of dichlorodiazadienes synthesized based on 4-methylbenzaldehyde, UNEC J. Eng. Appl. Sci. 5(1) (2025) 96-114. https://doi.org/10.61640/ujeas.2025.0511
Anyone you share the following link with will be able to read this content:

This article is licensed under the Creative Commons Attribution ( CC BY 4.0 ) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
N.Q. Shikhaliyev, M.L. Kuznetsov, A.M. Maharramov, A.V. Gurbanov, N.E. Ahmadova, V.G. Nenajdenko, A.J. Pombeiro, CrystEngComm 21(34) (2019) 5032. https://doi.org/10.1039/C9CE00956F
N.Q. Shikhaliyev, N.E. Ahmadova, A.V. Gurbanov, A.M. Maharramov, G.Z. Mammadova, V.G. Nenajdenko, A.J. Pombeiro, Dyes and Pigments 150 (2018) 377. https://doi.org/10.1016/j.dyepig.2017.12.033
N.G. Shikhaliyev, A.M. Maharramov, K.N. Bagirova, G.T. Suleymanova, B.D. Tsyrenova, V.G. Nenajdenko, A.G. Tskhovrebov, Mendeleev Communications 31(2) (2021) 191. https://doi.org/10.1016/j.mencom.2021.03.015
V.G. Nenajdenko, N.G. Shikhaliyev, A.M. Maharramov, K.N. Bagirova, G.T. Suleymanova, A.S. Novikov, A.G. Tskhovrebov, Molecules 25(21) (2020) 5013. https://doi.org/10.3390/molecules25215013
A.M. Maharramov, N.Q. Shikhaliyev, G.T. Suleymanova, A.V. Gurbanov, G.V. Babayeva, G.Z. Mammadova, A.J. Pombeiro, Dyes and Pigments 159 (2018) 135. https://doi.org/10.1016/j.dyepig.2018.06.022
A.Y. Sukhorukov, Frontiers in Chemistry 12 (2024) 1403024. https://doi.org/10.3389/fchem.2024.1403024
C. Curti, L. Battistini, A. Sartori, F. Zanardi, Chem. Rev. 120(5) (2020) 2448. https://doi.org/10.1021/acs.chemrev.9b00481
S.M.M. Lopes, A.L. Cardoso, A. Lemos, T.M.V.D. Pinho E. Melo, Chem. Rev. 118(23) (2018) 11324. https://doi.org/10.1021/acs.chemrev.8b00375
S.M. Weinreb, Synlett 30(16) (2019) 1855. https://doi.org/10.1055/s-0037-1611899
S.E. Denmark, S.T. Nguyen, R.Y. Baiazitov, Heterocycles 76(1) (2008) 143. https://doi.org/10.3987/com-08- s(n)15
Z.M. Png, H. Zeng, Q. Ye, J. Xu, Chem. Asian J. 12 (2017) 2142. http://dx.doi.org/10.3987/COM-08-S(N)21
R.Y. Baiazitov, S.E. Denmark, In Methods and applications of cycloaddition reactions in organic syntheses (2013) 471. https://doi.org/10.1002/9781118778173.ch16
K. Selvaraj, S. Chauhan, K. Sandeep, K.C.K. Swamy, Chem. Asian J. 15(16) (2020) 2380. https://doi.org/10.1002/asia.202000545
P.Y. Ushakov, S.L. Ioffe, A.Y. Sukhorukov, Organic Chemistry Frontiers 9(19) (2022) 5358. https://doi.org/10.1039/D2QO00698G
Y. Wang, Z. Jin, L. Zhou, X. Lv, Organic and Biomolecular Chemistry 22(2) (2024) 252. https://doi.org/10.1039/D3OB01626A
N.G. Shikhaliyev, A.M. Maharramov, G.T. Suleymanova, G.V. Babayeva, G.Z. Mammadova, I.M. Shikhaliyeva, V.G. Nenajdenko, Organic Chemistry (2021) 67. https://doi.org/10.3390/molecules27165110
K. Özkaraca, M. Akkurt, N.Q. Shikhaliyev, G.Z. Mammadova, D.M. Shadrack, Acta Crystallographica Section E: Crystallographic Communications 76(8) (2020) 1251. https://doi.org/10.1107/S2056989020009202
K. Özkaraca, M. Akkurt, N.Q. Shikhaliyev, I.M. Shikhaliyeva, A. Bhattarai, Acta Crystallographica Section E: Crystallographic Communications 76(6) (2020) 811. https://doi.org/10.1107/S2056989020006106
S. Balgotra, P.K. Verma, R.A. Vishwakarma, S.D. Sawant, Catalysis Reviews 62(3) (2020) 406. https://doi.org/10.1080/01614940.2019.1702191
J.A. Camarero, B.J. Hackel, J.J. De Yoreo, A.R. Mitchell, The Journal of organic chemistry 69(12) (2004) 4145. https://doi.org/10.1021/jo040140h
W. Kaim, Angewandte Chemie International Edition in English 22(3) (1983) 171. https://doi.org/10.1002/anie.198301713
S.H. Rohane, A.G. Makwana, Asian Journal of Research in Chemistry 10(4) (2017) 417. https://doi.org/10.5958/0974-4150.2017.00070.0
J. Buckingham, Quarterly Reviews, Chemical Society 23(1) (1969) 37. https://doi.org/10.1039/QR9692300037
T.A. Khattab, Materials Chemistry and Physics 254 (2020) 123456 https://doi.org/10.1016/j.matchemphys.2020.123456
O. Tšubrik, U. Mäeorg, R. Sillard, U. Ragnarsson, Tetrahedron 60(38) (2004) 8363. https://doi.org/10.1016/j.tet.2004.07.020
A. Hosseinian, R. Mohammadi, S. Ahmadi, A. Monfared, Z. Rahmani, RSC advances 8(59) (2018) 33828. https://doi.org/10.1039/C8RA06423G
S. Crespi, N.A. Simeth, B. König, Nature Reviews Chemistry 3(3) (2019) 133. https://doi.org/10.1038/s41570-019-0074-6
N.Q. Shikhaliyev, K. Özkaraca, M. Akkurt, X.N. Bagirova, G.T. Suleymanova, M.S. Abdulov, S. Mlowe, Acta Crystallographica Section E: Crystallographic Communications 77(11) (2021) 1158. https://doi.org/10.1107/S2056989021010756
Z. Atioğlu, M. Akkurt, N.Q. Shikhaliyev, G.T. Suleymanova, K.N. Bagirova, F.A. Toze, Acta Crystallographica Section E: Crystallographic Communications 75(2) (2019) 237. https://doi.org/10.1107/S2056989019000707
S.T. Çelikesir, M. Akkurt, N.Q. Shikhaliyev, N.A. Mammadova, G.T. Suleymanova, V.N. Khrustalev, A. Bhattarai, Acta Crystallographica Section E: Crystallographic Communications 78(4) (2022) 404. https://doi.org/10.1107/S205698902200278X
Z. Atioğlu, M. Akkurt, N.Q. Shikhaliyev, N.A. Mammadova, G.V. Babayeva, V.N. Khrustalev, A. Bhattarai, Acta Crystallographica Section E: Crystallographic Communications 78(8) (2022) 804. https://doi.org/10.1107/S2056989022007113
N.G. Shikhaliyev, G.T. Suleymanova, A.A. Israyilova, K.G. Ganbarov, G.V. Babayeva, K.A. Garazadeh, V.G. Nenajdenko, Organic Chemistry vi (2019) 0.
N.G. Shikhaliyev, U.F. Askerova, S.H. Mukhtarova, A.A. Niyazova, P.V. Dorovatovskii, V.N. Khrustalev, V.G. Nenajdenko, Russian Journal of Organic Chemistry 56 (2020) 185. https://doi.org/10.1134/S1070428020020013
N.G. Shikhaliev, G.T. Suleymanova, K.N. Bagirova, U.F. Asgerova, K.A. Garazadeh, G.V. Babayeva, N.E. Ahmedova, V.G. Nenajdenko, Journal of Low Dimensional Systems 2(2) (2018) 24.
G.M. Sheldrick, Acta Crystallographica Section A: Foundations and Advances 71(1) (2015) 3. https://doi.org/10.1107/S2053273314026370
K. Özkaraca, M. Akkurt, N.Q. Shikhaliyev, U.F. Askerova, G.T. Suleymanova, G.Z. Mammadova, D.M. Shadrack, Acta Crystallographica Section E: Crystallographic Communications 76(8) (2020) 1251. https://doi.org/10.1107/S2056989020009202
N.Q. Shikhaliyev, S.A. İbrahimova, G.T. Atakishiyeva, N.E. Ahmedova, G.V. Babayeva, V.N. Khrustalev, Z. Atioğlu, M. Akkurt, A. Bhattarai, Acta Crystallographica Section E: Crystallographic Communications 80(2) (2024) 184. https://doi.org/10.1107/S2056989024000732
P.R. Spackman, M.J. Turner, J.J. McKinnon, S.K. Wolff, D.J. Grimwood, D. Jayatilaka, M.A. Spackman, Journal of Applied Crystallography 54(3) (2021) 1006. https://doi.org/10.1107/S1600576721002910
V.R. Hathwar, M. Sist, M.R. Jørgensen, A.H. Mamakhel, X. Wang, C.M. Hoffmann, K. Sugimoto, B. Iversen, IUCrJ 2(5) (2015) 563. https://doi.org/10.1107/S2052252515012130
G.M. Sheldrick, Acta Crystallographica Section C: Structural Chemistry 71(1) (2015) 3. https://doi.org/10.1107/S2053229614024218
Rigaku OD CrysAlis PRO 1.171.43.92a. Rigaku Oxford Diffraction, Yarnton, England (2024).