UNEC Journal of Engineering and Applied Sciences Volume 5, No 1, pages 83-90 (2025) Cite this article, 803 https://doi.org/10.61640/ujeas.2025.0509
Oil and petroleum products are highly soluble in the aquatic environment and are among the most widespread pollutants of groundwater and surface water, posing a serious threat to global drinking water supplies and aquatic ecosystems [1].
Oil spills are a common occurrence in the environment, primarily because we use oil and oil products extensively in modern times. According to research, oil and oil products spill into the environment every day [1, 2]. Oil spillage in the environment, assessment of environmental damage, and selection of measures for cleaning oil from the environment are among the primary objectives of the modern era [1, 3].
Many cleaning processes are known for removing oil from water. In our research, we preferred the adsorption method and used the plant of origin waste as an adsorbent [1, 2]. The primary purpose of utilizing plant-based waste is to provide technology with minimal waste and a reduced environmental impact [2, 3].
The primary objective of implementing zero-waste and low-waste technologies is to substantially reduce the solid waste generated due to population growth, rapid economic development, and urbanization. In modern times, solid waste in the environment is one of the global environmental problems [1-3].
In general, the depletion of natural resources leads to an environmental crisis. To prevent the depletion of natural resources and to clean the environment of waste of natural origin, several measures are implemented for the strategic management of waste [1]. The primary objective of these measures is to minimize solid waste and promote the use of low-waste and zero-waste technologies.
For this purpose, in our research work, plant-based solid waste, specifically hazelnut shells, was reused and utilized in the treatment of water contaminated with oil and oil products [1, 2]. Additionally, to enhance the sorption capacity of hazelnut shells, their surface is coated with superparamagnetic Fe3O4 nanoparticles [2, 3]. By coating the surface of hazelnut shells with superparamagnetic nanoparticles, a novel type of bio-nanoadsorbent was synthesized. The primary purpose of selecting Fe3O4 nanoparticles is that they possess magnetic properties and are economically viable. Based on the experiments, it was determined that the newly synthesized bio-nanoadsorbent based on hazelnut shell+Fe3O4 nanoparticles is more effective in treating oil-polluted waters [2,3].
Fe3O4 magnetic nanoparticles were synthesized because of the co-precipitation of Fe3+ and Fe2+ ions [4]. After drying the hazelnut shell in a muffle furnace at a temperature of 600C for 24 hours, a biosorbent of plat of origin waste is synthesized by grinding and crushing [4-5]. Uncrushed hazelnuts are less absorbent than more crushed hazelnuts. This is because the reduced size of the hazelnut shells increases their surface area, resulting in a greater adsorption capacity compared to unshredded hazelnut shells. To enhance the activity and sorption capacity of the biosorbent, Fe3O4 superparamagnetic nanoparticles were impregnated onto the surface of the hazelnut shell under laboratory conditions [4-7]. For the synthesis of bio-nanoadsorbents based on hazelnut shells and Fe3O4 nanoparticles, varying concentrations of Fe3O4 nanoparticles (1%, 3%, 5%, and 10%) are added to the hazelnut shell and thoroughly mixed for a few minutes. Then, 1 ml of a 25% ammonium solution is added, and the mixture is mixed for 1 hour. Synthesized bio-nano adsorbents are washed in a centrifuge and then transferred into a Petri dish, where they are dried [7, 8]. The oil purification experiments of the newly synthesized bio-nanoadsorbent and the pure plant of origin of the biosorbent based on hazelnut shell+Fe3O4 nanoparticles were performed [8-10].
SEM and EDX analysis of biosorbents and bio-nanoadsorbents was performed on a ZEISS DSM 940A scanning electron microscope.
An experiment was conducted using the synthesized hazelnut shell biosorbent and bio-nanoadsorbent for the adsorption of oil-contaminated water [11-13]. First, an oily water sample was prepared by mixing 10 mL of oil with 100 mL of water. Then, biosorbent (hazelnut shell) and bio-nanoadsorbent (based on hazelnut shell + Fe3O4 nanoparticles) are added to the oil water for the adsorption experiment. An experiment was conducted to investigate the time dependence of the sorption process for biosorbents and bio-nanoadsorbents, with a focus on the amount of oil adsorbed. These experiments enable the determination of optimal conditions [13-15].
For the experiment, 0.5 g of hazelnut shell and hazelnut shell + Fe3O4 were taken, respectively. The experiment on the time dependence of biosorbent and bioadsorbent was carried out over 1, 2, 4, 6, 10, 12, 15, 20, 30, and 60 minutes.

Figure 1. Time dependence graph of a) Hazelnut shell and b)bio-nanoadsorbent synthesized based on hazelnut shell+ Fe3O4 nanoparticles
a)
According to the graphs of time dependence in figure 1, the biosorbent adsorbs 60.26% and the bio-nanoadsorbent 92.1% of oil in 20 minutes. According to figure 1 a,b, 0.5 g of hazelnut shell adsorbs 61.25% oil in 30 minutes, and the adsorbent we synthesized based on hazelnut shell+Fe3O4 nanoparticles, which we use as a bio-nanoadsorbent, adsorbs 92.5% oil in 13 minutes, and these results show the optimal time.

Figure 2. a) HN and b) HN+ Fe3O4 pH dependence graph of a bio-nanoadsorbent synthesized based on nanoparticles
We then continued our experiment to determine the pH dependence of the bio-nanoadsorbent synthesized based on the biosorbent and hazelnut shell + Fe3O4 nanoparticles. In the experiment, the sorption process was carried out over a pH range of 0-10 for both the biosorbent and bio-nanoadsorbent [16]. Based on the results obtained, it was determined that the optimal pH for oil absorption by biosorbent and bio-nanoadsorbent is 7.5 (figure 2 a,b). Shahabaldin Rezania [17] et al. reported in their paper that Fe2O3 nanoparticles are effective adsorbents for heavy metal ions and that bioreduced α-Fe2O3 removes 82.8%, 47.6%, and 75.7% of COD, TDS, and TSS from textile wastewater, respectively. Mansooreh khalatbary et al. reported the adsorption of malachite green from aqueous solutions using γ-Fe2O3/MWCNTs/cellulose adsorbent obtained from waste tires and natural cellulose and determined that the malachite green adsorption capacity of γ-Fe2O3/MWCNTs/cellulose was 47.61 mg/g (87.35%) [18].
The experiment was also conducted at temperatures of 100C, 21.50C (room temperature), and 400C [19-22].

Figure 3. a) HN and b) HN+ Fe3O4 temperature dependence graph of a bio-nanoadsorbent synthesized based on nanoparticles (1-10ºC, 2-21.5ºC, 3-40ºC)
Figure 3 a,b shows that the optimal temperature for oil adsorption of bio-nanoadsorbent synthesized based on hazelnut shell and hazelnut shell + Fe3O4 nanoparticles is considered to be 100C and room temperature (21.50C) [22]. Thus, as the temperature rises, the adsorption decreases.
Initially, a scanning electron microscopic (SEM) analysis of bio-nanoadsorbent based on hazelnut shell and newly synthesized hazelnut shell+Fe3O4 nanoparticles was performed to determine the coverage of the hazelnut shell surface by nanoparticles [23-25]. During the analysis, high-quality images of the surface of both types of adsorbents were obtained. Figure 4 a and b show SEM images of bionanoadsorbents synthesized using hazelnut shells and hazelnut shell+Fe3O4 nanoparticles [26].
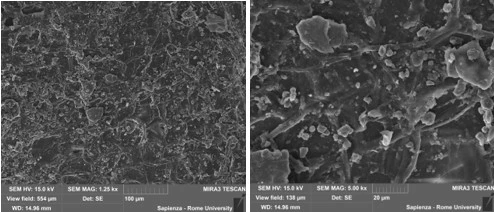
Figure 4. SEM micrographs of hazelnut shell a) and hazelnut shell+Fe3O4 b) bionanoadsorbents at different magnifications
During the SEM analysis of the hazelnut shell, which was used as a pure plant-based adsorbent, voids and pores were observed on the surface of the bioadsorbent. The results of the SEM analysis of the bio-nanoadsorbent revealed that the sizes were further reduced, as nanoparticles covered the surface of the hazelnut shell pores and penetrated into the interior from the surface of the hazelnut shell [27]. According to the SEM image, it is clear that the morphology of the nanoparticles is quite spherical, with a homogeneous distribution and very small sizes. In contrast, the bio-nanoadsorbent is heterogeneously distributed, together with the collected nanoparticles and hazelnut shell [27].
Elemental EDX analysis was performed to determine the elemental composition and chemical characteristics of the pure plant-derived biosorbent and the hazelnut shell+Fe3O4 bio-nanoadsorbent [28-30]. Energy-dispersive X-ray analysis (EDX), a technique that can be combined with scanning electron microscopy (SEM) analysis, enables elemental analysis over areas as small as a few nanometers using electron microscopes.
The results of energy-dispersive X-ray (EDX) analysis, based on the bionanoadsorbent hazelnut shell and Fe3O4, are presented in figure 5 a,b. The chemical composition of the hazelnut shell reveals the presence of primary elements, including C, K, O, and Cl [30-31]. In addition, in figure 5b, the presence of Fe, Na, and Au residues, along with C, O, and Cl, was observed on the surface of the bio-nanoadsorbent synthesized using hazelnut shell+Fe3O4 nanoparticles in complex shapes. In both types of samples, the element carbon is observed as a significant component deposited on the machined surface, either in its free form or as a compound due to decomposition [32-34].
Based on the results shown in table 1, it can be determined that although chlorides are present in the chemical composition of the hazelnut shell surface, oxygen is the dominant element.
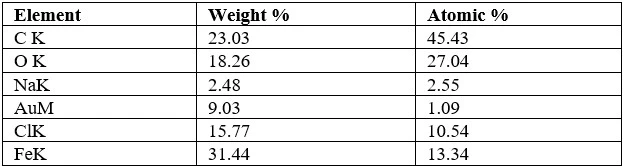
Table 2. Results of EDX analysis of newly synthesized bio-nanoadsorbent based on hazelnut shell+Fe3O4 nanoparticles
Based on the results shown in table 2, it can be determined that although chlorides are present in the chemical composition of the bio-nanoadsorbent's surface, iron (Fe) is observed as the dominant element [34]. According to this result, it is determined that Fe3O4 nanoparticles were located on the surface of the hazelnut shell. Thus, the analysis of the chemical composition of the bio-nanoadsorbent's surface enables the determination of the presence of chlorides in addition to the Fe element. This will allow us to confirm the absorption of Fe3O4 nanoparticles on the surface of the hazelnut shell, as it corresponds to the chemical composition of the substances used in the synthesis of Fe3O4 superparamagnetic nanoparticles [34-{ref:35}].
The hazelnut shell biosorbent, which we utilize as a waste product from our plant, was synthesized in our research work to provide low-waste and zero-waste technology. The activity of the biosorbent in purifying water polluted by oil and petroleum products was tested, and new bionanoadsorbents were synthesized by impregnating Fe3O4 superparamagnetic nanoparticles onto the surface of the biosorbent to increase its adsorption capacity. As a result of the oil sorption of the hazelnut shell, it was determined that the biosorbent adsorbed 61.25% of the oil within 30 minutes. The adsorption capacity of bio-nanoadsorbent is 92.5% (13 minutes). The optimal pH for both adsorbents is considered to be 7.5, and the optimal temperatures are 10 and 21.50 C. Based on the SEM and EDX analyses of the synthesized natural biosorbent and bio-nanoadsorbent, it was determined that Fe3O4 nanoparticles agglomerated inside the hazelnut shells. Thus, according to SEM and EDX analysis results, Fe3O4 superparamagnetic nanoparticles wholly covered the surface of the hazelnut shell.
1 F. Ferrero, J. Hazard. Mater. 142 (2007) 144. https://doi.org/10.1016/j.jhazmat.2006.07.072
2 A. Bhatnagar, M. Sillanpää, A review. Chem. Eng. J. 157 (2010) 277. https://doi.org/10.1016/j.cej.2010.01.007
3 M. Ramazanov, H. Shirinova, F. Hajiyeva, H.M. Mamedov, Mater. Chem. Phys. 253(1) (2020) 123287. https://doi.org/10.1016/j.matchemphys.2020.123287
4 M. Ramazanov, H. Shirinova, F. Hajiyeva, et al. Int. J. Mod. Phys. B 33(10) (2019) 1950083. http://dx.doi.org/10.1142/S0217979219500838
5 M. Ramazanov, A. Maharramov, R. Ali-zada, H. Shirinova, F. Hajiyeva, J. Theor. Appl. Phys. 12(1) (2018) 7. http://dx.doi.org/10.1007/s40094-018-0282-3
6 M. Ramazanov, A. Maharramov, F. Hajiyeva, H. Shirinova, L.D. Palma, J Inorg Organomet Polym Mater. 28(3) (2018) 1171. https://link.springer.com/article/10.1007/s10904-017-0767-6
7 М.A. Ramazanov, A.M. Maharramov, L.D. Palma, F.V. Hajiyeva, H.A. Shirinova, Ferroelectr. 537(1) (2018) 191. https://doi.org/10.1080/00150193.2018.1528943
8 М.А. Ramazanov, H.A. Shirinova, F.V. Hajiyeva, A. Karimova, Integr. Ferroelectrics 201(1) (2019) 218. https://doi.org/10.1080/10584587.2019.1668712
9 M. Ramazanov, H. Shirinova, F. Hajiyeva, A. Karimova, Int. J. Mod. Phys. B 33(27) (2019) 1950315. http://dx.doi.org/10.1142/S0217979219503156
10 W. Yang, J. Wang, X. Shi, H. Tang, X. Wang, S. Wang, W. Zhang, J. Lu, Ind. Eng. Chem. Res. 59 (2020) 5194. http://dx.doi.org/10.1021/acs.iecr.0c00003
11 M.A. Al-Ajji, M.A. Al-Ghouti, J. Water Process Eng. 44 (2021) 102354. https://doi.org/10.1016/j.jwpe.2021.102354
12 Y. Liu, Y. Zhao, W. Cheng, T. Zhang, J. Colloid Interface Sci. 579 (2020) 766. https://doi.org/10.1016/j.jcis.2020.06.083
13 K. Belkassa, M. Khelifa, I. Batonneau-Gener, K. Marouf-Khelifa, A. Khelifa, J. Hazard. Mater. 415 (2021) 125656. https://doi.org/10.1016/j.jhazmat.2021.125656
14 L.D. Ardila-Leal, R.A. Poutou-Piñales, A.M. Pedroza-Rodríguez, B.E. Quevedo-Hidalgo, Molecules, 26 (2021) 3813. https://doi.org/10.3390/molecules26133813
15 A. Djelad, A. Mokhtar, A. Khelifa, A. Bengueddach, M. Sassi, Int. J. Biol. Macromol. 139 (2019) 944. https://doi.org/10.1016/j.ijbiomac.2019.08.068
16 Sh. Rezania, N. Darajeh, P.F. Rupani, A. Mojiri, H. Kamyab and M. Taghavijeloudar, Applied Sciences 14(24) (2024) 11492. https://doi.org/10.3390/app142411492
17 M. Khalatbary, M.H. Sayadi, M. Hajiani and M. Nowrouzi, Biomass Conversion and Biorefinery 14(2) (2024) 1. http://dx.doi.org/10.1007/s13399-022-02475-4 http://dx.doi.org/10.1007/s13399-022-02475-4
18 X. Pang, L. Sellaoui, D. Franco, G. Dotto, J. Georgin, A. Bajahzar, H. Belmabrouk, A.B. Lamine, A. Bonilla-Petriciolet, Z. Li, Chemical Engineering Journal 378 (2019) 122101. https://doi.org/10.1016/j.cej.2019.122101
19 L.H. Nguyen, H.T. Nguyen, B.Q.G. Le, M.H.D. Dang, T.T.T. Nguyen, N.X.D. Mai, T.L.H. Doan, Colloid and Interface Science Communications 46 (2021). http://dx.doi.org/10.1016/j.colcom.2021.100511
20 P. Moharrami, E. Motamedi, Bioresour. Technol. 313 (2020) 123661. http://dx.doi.org/10.1016/j.biortech.2020.123661
21 M. Ahmed, F. Mashkoor, A. Nasar, Groundw. Sustain. Dev. 10 (2020) 100322. http://dx.doi.org/10.1016/j.gsd.2019.100322
22 S. Bayil Oguzkan, S. Karadeniz, B. Karagül, A. Uzun, E. Sine Aksoy, Ö. Özensoy Güler, Ü. Çakir, H.I. Ugras, Int. J. Pharmacol. 14 (2018) 835. https://doi.org/10.3923/ijp.2018.835.840
23 W. Yang, J. Wang, X. Shi, H. Tang, X. Wang, S. Wang, W. Zhang, J. Lu, Ind. Eng. Chem. Res. 59 (2020) 5194. http://dx.doi.org/10.1021/acs.iecr.0c00003
24 I. Al Zubaidi, M. Al Zubaidi, M. Tajik, M. Al Zubaidi, M. Al Mutairi, M. Sheikh, Y. Chen, M. Al-Yasiri, A. Alsudays, Proceedings of the 5th International Conference of Fluid Flow, Heat and Mass Transfer (FFHMT'18), Niagara Falls, Canada (2018) 159-1. https://doi.org/10.11159/FFHMT18.159
25 S.M. Shartooh, S.M. Shartooh, S.A.K. Al-Hiyaly, Iraqi Journal of Science 54(4) (2013) 823
26 A. Hashem, A.J. Fletcher, M. El-Sakhawy, L.A. Mohamed, S. Farag, J Polym Environ. 28(9) (2020) 2498. https://link.springer.com/article/10.1007/s10924-020-01789-6
27 N. Bellahsen, G. Varga, N. Halyag, S. Kertész, E. Tombacz, C. Hodur, Int J Environ Sci Technol 18 (2021) 711. http://dx.doi.org/10.1007/s13762-020-02863-1
28 J. Lopez-Luna, L.E. Ramirez-Montes, S. MartinezVargas, A.I. Martinez, O.F. Mijangos-Ricardez, A. del Carmen, M. González-Chávez, R. Carrillo-González, F.A. Solís-Domínguez, M. del Carmen CuevasDíaz, V. Vázquez-Hipólito, SN Appl Sci 1(950) (2019) 1.
29 J. Sahadevan, R. Sojiya, N. Padmanathan, K. Kulathuraan, M.G. Shalini, P. Sivaprakash, E. Muthu, Materials Today Proceedings 58(3) (2022) 895. http://dx.doi.org/10.1016/j.matpr.2021.11.420
30 A. Hashem, C.O. Aniagor, M.F. Nasr, A. Abou-Okeil, Int J Biol Macromol 176 (2021) 201. https://doi.org/10.1016/j.ijbiomac.2021.02.067
31 A. Boukir, S. Fellak, P. Doumenq, Heliyon 5(9) (2019) e02477.
32 C. Aniagor, E. Abdel-Halim, A. Hashem, J Environ Chem Eng 9(4) (2021) 105703. https://doi.org/10.1016/j.jece.2021.105703
33 C.O. Aniagor, M. Afifi, A. Hashem, Carbohyd Polym Technol Appl 2 (2021) 100141. http://dx.doi.org/10.1016/j.carpta.2021.100141
34 A. Hashem, C.O. Aniagor, M.A.-F. Afifi, A. Abou-Okeil, S.H. Samaha, Korean J Chem Eng 38(10) (2021) 2157. https://doi.org/10.1007/s11814-021-0856-7
U.N. Naghiyeva, S.R. Hajiyeva, F.V. Hajiyeva, SEM analysis of new bio-nanoadsorbents synthesized based on hazelnut shell and magnetite nanoparticles, UNEC J. Eng. Appl. Sci. 5(1) (2025) 83-90. https://doi.org/10.61640/ujeas.2025.0509
Anyone you share the following link with will be able to read this content:

This article is licensed under the Creative Commons Attribution ( CC BY 4.0 ) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
F. Ferrero, J. Hazard. Mater. 142 (2007) 144. https://doi.org/10.1016/j.jhazmat.2006.07.072
A. Bhatnagar, M. Sillanpää, A review. Chem. Eng. J. 157 (2010) 277. https://doi.org/10.1016/j.cej.2010.01.007
M. Ramazanov, H. Shirinova, F. Hajiyeva, H.M. Mamedov, Mater. Chem. Phys. 253(1) (2020) 123287. https://doi.org/10.1016/j.matchemphys.2020.123287
M. Ramazanov, H. Shirinova, F. Hajiyeva, et al. Int. J. Mod. Phys. B 33(10) (2019) 1950083. http://dx.doi.org/10.1142/S0217979219500838
M. Ramazanov, A. Maharramov, R. Ali-zada, H. Shirinova, F. Hajiyeva, J. Theor. Appl. Phys. 12(1) (2018) 7. http://dx.doi.org/10.1007/s40094-018-0282-3
M. Ramazanov, A. Maharramov, F. Hajiyeva, H. Shirinova, L.D. Palma, J Inorg Organomet Polym Mater. 28(3) (2018) 1171. https://link.springer.com/article/10.1007/s10904-017-0767-6
М.A. Ramazanov, A.M. Maharramov, L.D. Palma, F.V. Hajiyeva, H.A. Shirinova, Ferroelectr. 537(1) (2018) 191. https://doi.org/10.1080/00150193.2018.1528943
М.А. Ramazanov, H.A. Shirinova, F.V. Hajiyeva, A. Karimova, Integr. Ferroelectrics 201(1) (2019) 218. https://doi.org/10.1080/10584587.2019.1668712
M. Ramazanov, H. Shirinova, F. Hajiyeva, A. Karimova, Int. J. Mod. Phys. B 33(27) (2019) 1950315. http://dx.doi.org/10.1142/S0217979219503156
W. Yang, J. Wang, X. Shi, H. Tang, X. Wang, S. Wang, W. Zhang, J. Lu, Ind. Eng. Chem. Res. 59 (2020) 5194. http://dx.doi.org/10.1021/acs.iecr.0c00003
M.A. Al-Ajji, M.A. Al-Ghouti, J. Water Process Eng. 44 (2021) 102354. https://doi.org/10.1016/j.jwpe.2021.102354
Y. Liu, Y. Zhao, W. Cheng, T. Zhang, J. Colloid Interface Sci. 579 (2020) 766. https://doi.org/10.1016/j.jcis.2020.06.083
K. Belkassa, M. Khelifa, I. Batonneau-Gener, K. Marouf-Khelifa, A. Khelifa, J. Hazard. Mater. 415 (2021) 125656. https://doi.org/10.1016/j.jhazmat.2021.125656
L.D. Ardila-Leal, R.A. Poutou-Piñales, A.M. Pedroza-Rodríguez, B.E. Quevedo-Hidalgo, Molecules, 26 (2021) 3813. https://doi.org/10.3390/molecules26133813
A. Djelad, A. Mokhtar, A. Khelifa, A. Bengueddach, M. Sassi, Int. J. Biol. Macromol. 139 (2019) 944. https://doi.org/10.1016/j.ijbiomac.2019.08.068
Sh. Rezania, N. Darajeh, P.F. Rupani, A. Mojiri, H. Kamyab and M. Taghavijeloudar, Applied Sciences 14(24) (2024) 11492. https://doi.org/10.3390/app142411492
M. Khalatbary, M.H. Sayadi, M. Hajiani and M. Nowrouzi, Biomass Conversion and Biorefinery 14(2) (2024) 1. http://dx.doi.org/10.1007/s13399-022-02475-4 http://dx.doi.org/10.1007/s13399-022-02475-4
X. Pang, L. Sellaoui, D. Franco, G. Dotto, J. Georgin, A. Bajahzar, H. Belmabrouk, A.B. Lamine, A. Bonilla-Petriciolet, Z. Li, Chemical Engineering Journal 378 (2019) 122101. https://doi.org/10.1016/j.cej.2019.122101
L.H. Nguyen, H.T. Nguyen, B.Q.G. Le, M.H.D. Dang, T.T.T. Nguyen, N.X.D. Mai, T.L.H. Doan, Colloid and Interface Science Communications 46 (2021). http://dx.doi.org/10.1016/j.colcom.2021.100511
P. Moharrami, E. Motamedi, Bioresour. Technol. 313 (2020) 123661. http://dx.doi.org/10.1016/j.biortech.2020.123661
M. Ahmed, F. Mashkoor, A. Nasar, Groundw. Sustain. Dev. 10 (2020) 100322. http://dx.doi.org/10.1016/j.gsd.2019.100322
S. Bayil Oguzkan, S. Karadeniz, B. Karagül, A. Uzun, E. Sine Aksoy, Ö. Özensoy Güler, Ü. Çakir, H.I. Ugras, Int. J. Pharmacol. 14 (2018) 835. https://doi.org/10.3923/ijp.2018.835.840
W. Yang, J. Wang, X. Shi, H. Tang, X. Wang, S. Wang, W. Zhang, J. Lu, Ind. Eng. Chem. Res. 59 (2020) 5194. http://dx.doi.org/10.1021/acs.iecr.0c00003
I. Al Zubaidi, M. Al Zubaidi, M. Tajik, M. Al Zubaidi, M. Al Mutairi, M. Sheikh, Y. Chen, M. Al-Yasiri, A. Alsudays, Proceedings of the 5th International Conference of Fluid Flow, Heat and Mass Transfer (FFHMT'18), Niagara Falls, Canada (2018) 159-1. https://doi.org/10.11159/FFHMT18.159
S.M. Shartooh, S.M. Shartooh, S.A.K. Al-Hiyaly, Iraqi Journal of Science 54(4) (2013) 823
A. Hashem, A.J. Fletcher, M. El-Sakhawy, L.A. Mohamed, S. Farag, J Polym Environ. 28(9) (2020) 2498. https://link.springer.com/article/10.1007/s10924-020-01789-6
N. Bellahsen, G. Varga, N. Halyag, S. Kertész, E. Tombacz, C. Hodur, Int J Environ Sci Technol 18 (2021) 711. http://dx.doi.org/10.1007/s13762-020-02863-1
J. Lopez-Luna, L.E. Ramirez-Montes, S. MartinezVargas, A.I. Martinez, O.F. Mijangos-Ricardez, A. del Carmen, M. González-Chávez, R. Carrillo-González, F.A. Solís-Domínguez, M. del Carmen CuevasDíaz, V. Vázquez-Hipólito, SN Appl Sci 1(950) (2019) 1.
J. Sahadevan, R. Sojiya, N. Padmanathan, K. Kulathuraan, M.G. Shalini, P. Sivaprakash, E. Muthu, Materials Today Proceedings 58(3) (2022) 895. http://dx.doi.org/10.1016/j.matpr.2021.11.420
A. Hashem, C.O. Aniagor, M.F. Nasr, A. Abou-Okeil, Int J Biol Macromol 176 (2021) 201. https://doi.org/10.1016/j.ijbiomac.2021.02.067
A. Boukir, S. Fellak, P. Doumenq, Heliyon 5(9) (2019) e02477.
C. Aniagor, E. Abdel-Halim, A. Hashem, J Environ Chem Eng 9(4) (2021) 105703. https://doi.org/10.1016/j.jece.2021.105703
C.O. Aniagor, M. Afifi, A. Hashem, Carbohyd Polym Technol Appl 2 (2021) 100141. http://dx.doi.org/10.1016/j.carpta.2021.100141
A. Hashem, C.O. Aniagor, M.A.-F. Afifi, A. Abou-Okeil, S.H. Samaha, Korean J Chem Eng 38(10) (2021) 2157. https://doi.org/10.1007/s11814-021-0856-7