UNEC Journal of Engineering and Applied Sciences Volume 5, No 1, pages 115-123 (2025) Cite this article, 841 https://doi.org/10.61640/ujeas.2025.0512
The rapid growth of industrial production poses a significant threat to the environment. Key contributors to this impact include the generation of large volumes of mining and mineral processing waste, gas-air emissions, and industrial wastewater discharges. As production volumes and the diversity of manufactured goods increase, both the quantity and complexity of industrial wastewater also rise.
Equally pressing is the global shortage of clean freshwater, which is essential not only for human consumption but also for various industrial processes such as recycling, cooling, steam generation, and heating. In line with the internationally recognized principles of sustainable development, modern industrial enterprises increasingly aim to minimize wastewater discharge through the implementation of closed-loop water systems and to maximize the recycling of production and consumption waste. These initiatives align with the broader goal of transitioning to a circular economy [1–2].
Ongoing efforts to improve the environmental performance of electrochemical industries include the adoption of "green" electrolytes [3–5], the use of advanced reagents for wastewater treatment [6–9], the development of technologies for deep removal of heavy metal ions from water [10–15], and the establishment of closed water cycles to reduce both freshwater consumption and pollutant discharge [16–17].
A significant body of research focuses on wastewater treatment in the metallurgical and related industries, particularly on the removal of heavy and nonferrous metal compounds [18–21]. Due to their high toxicity, bioaccumulative nature, and teratogenic and carcinogenic effects, the discharge of untreated wastewater containing these metals into municipal sewage systems or natural water bodies is unacceptable [22–23].
An additional benefit of treating metal-laden wastewater is the potential for resource recovery. The extracted metals can be reused in production or sold to recycling facilities, offering economic as well as environmental advantages.
Conventional treatment of heavy metal-containing wastewater typically involves physicochemical methods such as neutralization, precipitation, and reduction, often combined with coagulation and flocculation. These reagent-based methods are capable of removing 95–99% of poorly soluble metal compounds and associated pollutants [24–27]. In certain cases, electrochemical, adsorption, or biological methods may be applied to target specific contaminants [28–32]. For achieving very low metal concentrations—suitable, for example, for fishery standards—advanced processes such as reverse osmosis, electrodialysis, or ion exchange may be employed [33–34].
Among the emerging technologies, electroflotation has shown great promise for treating wastewater with high concentrations of metal ions. It is particularly effective in removing poorly soluble metal compounds, petroleum products, and surfactants [35–37].
The objective of this study is to assess the influence of pH and background electrolytes on the electroflotation efficiency for removing poorly soluble compounds of heavy and nonferrous metals—specifically, binary mixtures of Cu-Zn, Ni-Zn, and Cu-Ni—from aqueous solutions, both in the absence and presence of various organic additives.
To study the flotation process, a laboratory unit with insoluble ORTA anodes and stainless steel cathodes was used. The DC source was a power supply unit HY 1803D (Japan), volume of the unit 500 ml, cross-sectional area (electrode surface area) 10 cm2, height of the liquid column 80 cm.
The model system was prepared by dissolving metal salts (CuSO4•5H2O; NiSO4•7H2O; ZnSO4•7H2O; CoSO4•7H2O; FeSO4•7H2O) in distilled water. Salts (Na2SO4, NaNO3, NaCl, Na3PO4, Na2CO3) with a concentration of 1 mg/l were used as a background electrolyte. The pH correction was carried out with 1% NaOH solution.
The additives that are widely used in surface treatment of nonferrous metals and alloys were selected for the study. The data on their composition are presented in table 1.
Experimental conditions: С(Zn2+) = 50 mg/l, C(Cu2+) = 50 mg/l, C(Ni2+) = 50 mg/l, C(salt)=500 mg/l, Jv = 0.4 A/l, pH=10.0±0.1, process time 20 min.
The concentration of metal ions was determined by atomic adsorption spectroscopy and atomic emission spectroscopy with magnetic plasma [27, 38].
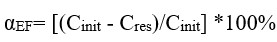
where Сinit, Сres are initial and final (residual) concentrations of metals in water, mg/dm3.
Figure 1 shows the data on the impact of pH on the efficiency of electroflotation removal of metals from the model system with Na2SO4 background electrolyte at different pH values.
The data in the diagram show that the removal efficiency of iron compounds, on average, does not exceed 75% and significantly (2.0 times) decreases at pH 11.0. The maximum efficiency (more than 96%) of removal of nickel, zinc, copper, and cobalt compounds is observed at pH 9.0-10.0, which agrees well with the data on the formation of hardly soluble forms of metal hydroxides. A further increase in pH leads to the beginning of dissolution of amphoteric metal bases, reduction of removal efficiency by 40-55%, and reverse transition of metals into solution. The alkaline medium has the least effect on the removal efficiency of cobalt compounds; the difference does not exceed 20%.
Figure 2 shows the results of electroflotation removal of metals from the model system with NaCl background electrolyte.
In the system with a chloride anion, the electroflotation removal of heavy metals from aqueous media is significantly intensified. The minimum efficiency (95%) is observed for iron compounds, which is due to the process of oxidation of iron (II) compounds in alkaline medium at high oxygen content with the formation of intermediate phases of iron (II, III) characterized by particle size of less than 1 micron, which significantly complicates the process of their adhesion with gas bubbles.
At pH 9.0, a slight decrease in the removal efficiency of all cations is observed, which is probably due to the formation of finely dispersed intermediates with reduced adhesion to gas-air bubbles. The presence of a chloride anion in the system significantly inhibits the processes of hydroxide dissolution at pH greater than 11.0.
The impact of pH on the efficiency of electroflotation removal of metals from the model system in the presence of NaNO3 as a background electrolyte differs slightly from the two previous experiments (figure 3).
This system is the least effective for the removal of iron (II) ions, which is associated with the process of its oxidation; the maximum result with an efficiency of 80% is achieved at pH = 10. Ni, Zn, Co, Cu cations in the presence of nitrate anion are removed almost completely from aqueous solutions, while similar to chloride-containing system inhibition of hydroxide dissolution processes at pH greater than 11.0 is observed.
A significant difference from the first three considered systems is characteristic of the background electrolyte Na3PO4 (figure 4).
The phosphate anion leads to a sharp inhibition of the electroflotation process (by 40 - 50 %) for copper, nickel, zinc, and iron in weakly alkaline medium (pH 8.0-9.0), which can be explained by the mechanism of dispersed phase release: at these pH values, mainly metal hydroxides are formed; the increase in the pH of the medium leads to the formation of hardly soluble metal phosphates, which apparently have extremely low adhesion to gas-air bubbles. At pH=11 the process of removal of all investigated metals stops completely (efficiency less than 10 %).
Figure 5 presents data on the impact of pH on the efficiency of electroflotation removal of metals from the model system with the background electrolyte Na2СO3.
The presence of the carbonate anion has no significant effect on the electroflotation process, and the achieved removal rates of hardly soluble metal compounds were comparable to chloride, sulfate, and nitrate systems in the range of pH 10-11.
The maximum removal efficiency for all cations was observed at pH 10.0, which probably corresponds to the formation of the least soluble form of metal carbonates. The sharp jump in electroflotation efficiency for cobalt compounds at pH 10.0 can be explained by hydration of the cobalt carbonate surface and an increase in the degree of its adhesion to gas-air bubbles.
Primary water purified by electroflotation subjected to post-treatment can be directed to municipal deep biological treatment facilities or reused for technological purposes (recycled water supply) [24- 25].
At higher pH levels (particularly around pH 10.0), metal ions such as Cu²⁺, Ni²⁺, and Zn²⁺ readily form insoluble hydroxides. These hydroxide precipitates exhibit favorable aggregation properties and increased hydrophobicity, which enhances their attachment to rising gas bubbles during electroflotation. Additionally, higher pH promotes a more stable and voluminous gas generation at the electrodes, improving the flotation and removal of particulate matter.
Regarding organic additives, we have added mechanistic details on the role of ethanol present in the PL-1 purifying liquid. Ethanol can modify the interfacial tension and alter the surface properties of metal hydroxide particles, potentially decreasing their hydrophobicity and reducing their adhesion to gas bubbles. This may explain the observed decrease in removal efficiency when PL-1 is introduced. In contrast, other additives such as penetrants may promote surface activation or stabilization of dispersions, thus enhancing flotation under certain conditions.
Figures 6-9 present data on the efficiency of electroflotation removal of metal ions (double co-presence) depending on the type of the organic additive used. In all experiments of the considered cation pairs, the increase in the additive concentration above 10 mg/l did not give additional increase in the purification efficiency, but created secondary water contamination by organic substances.
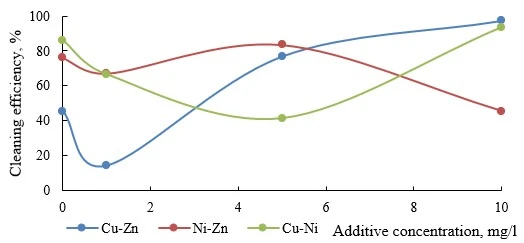
Figure 6. Efficiency of electroflotation removal of metal pairs depending on the concentration of organic additive PL-1
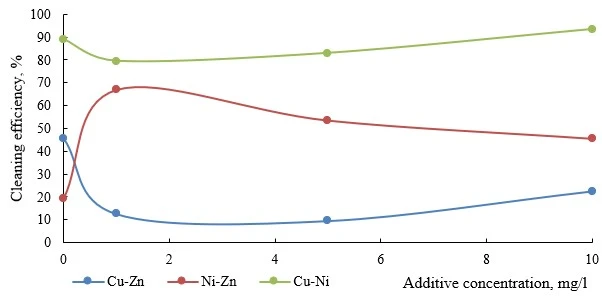
Figure 7. Efficiency of electroflotation removal of metal pairs depending on the concentration of organic penetrant additive
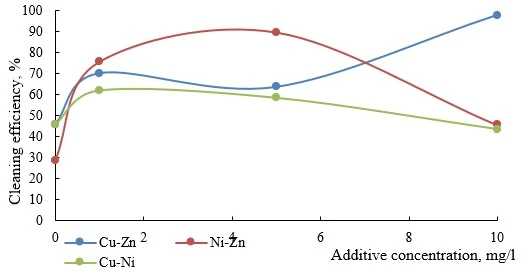
Figure 8. Efficiency of electroflotation removal of metal pairs depending on the concentration of organic varnish additive
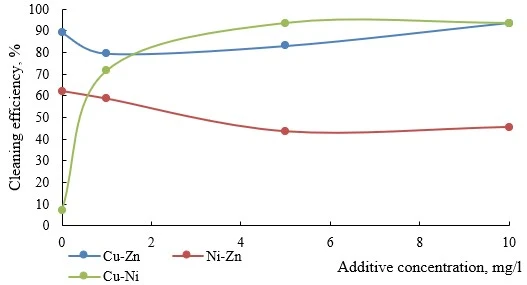
Figure 9. Efficiency of electroflotation removal of metal pairs depending on the concentration of organic solvent additive
The graph shows (figure 6) that an insignificant amount of the additive (1 mg/l) has a negative effect on the electroflotation removal of all metal pairs. When the concentration of the additive is increased, the purification efficiency significantly increases in all systems and is especially pronounced for the Cu-Ni pair (2-fold increase in removal efficiency).
The addition of penetrant in any amount negatively affects the electroflotation removal efficiency of the Cu-Zn pair (figure 7). For the Cu-Ni pair, the removal rate was maximal at an additive concentration of 10 mg/l, whereas lower concentrations of the penetrant led to a slight decrease in the pollutant removal efficiency. In the system Ni-Zn, even the minimum additive allowed an almost 3.5 times greater removal efficiency, but a further increase in the concentration of organic additive gradually reduced the purification efficiency from 75% to 50%. The results showed p-values > 0.05, indicating no significant difference between the purification efficiencies at 10 mg/L and higher concentrations.
In all studied systems, the introduction of varnish makes it possible to significantly increase the efficiency of electroflotation removal (figure 8). For nickel-containing pairs (Cu-Ni and Ni-Zn), flotation efficiency initially increased (2.5 times for Ni-Zn and 1.5 times for Cu-Ni), but a further increase of varnish concentration leads to a negative result.
As can be seen from the data in figure 9, the addition of solvent has a negative effect on the efficiency of electroflotation removal of Ni-Zn and Cu-Zn pairs. For Cu-Ni system, a pronounced increase in efficiency (8 times) was observed; the maximum result was achieved in the range of additive concentrations 5-10 mg/l.
Significant differences in the influence of various organic additives on the efficiency of electroflotation removal of metal mixtures can be explained as follows.
The components of PL-1 (figure 6) are non-ionogenic surfactants, which significantly inhibit the electroflotation processes [39-40]. Ethanol, which is part of the purifying liquid, is able to adsorb on the surfaces of copper and nickel hydroxide, increasing their hydrophilicity, which also interferes with the efficiency of the process [39].
The penetrant based on hydrophobic ditolyl methane in a hydrophilic butyl alcohol solution is characterized by a low degree of adsorption on the surface of nickel and copper hydroxides, and therefore has no significant effect on the flotation efficiency [40]. Zinc-containing pairs, probably, on the contrary, are exposed to the penetrant (high degree of adsorption) and the degree of removal for these systems significantly increased (figure 7).
For varnish, the increase in flotation efficiency is primarily due to the binding effect of phenol-formaldehyde component (a process similar to flocculation), aggregation of particles and their increased hydrophobicity, since the resin is lyophobic (figure 8). The presence of ethanol in the solution has an inhibitory effect on the flotation process.
In systems with a complex composition solvent based on butyl acetate, acetone, and xylene there are competing processes of adsorption on the surface of particles of hydroxides ofhydrophilic and hydrophobic components, which, depending on the metal-containing pair, can have no effect or, conversely, significantly intensify the process.
An analysis of the influence of anionic composition on electroflotation efficiency for metal ion removal shows that chloride-containing systems are the most favorable, ensuring stable flotation with ~98% removal at pH 10.0. Nitrate systems perform similarly, except for Fe, which shows ~80% removal. Sulfate-containing media are also effective, especially within pH 8.0–10.0, avoiding the formation of soluble amphoteric hydroxides. Under these conditions, metals like Zn, Cu, Ni, and Co can be nearly completely removed. Carbonate media also demonstrate high efficiency—over 95% for Zn, Cu, Fe, and Co, and ~90% for Ni. Sodium carbonate is both cost-effective and a strong alkalizing agent, with an optimal pH of 10.0. In contrast, phosphate anions severely inhibit electroflotation, likely due to the formation of stable, non-floatable complexes. Thus, phosphate-containing surfactants and phosphorus-based compounds should be avoided. The second part of the study examined the effect of organic additives on electroflotation of metal ion mixtures (Cu-Zn, Ni-Zn, Cu-Ni). In the Cu-Ni system, a solvent improved removal more than threefold, while a purifying liquid reduced it; varnish and penetrant had little effect. Zn-containing systems responded variably: the solvent had minimal impact, but varnish improved efficiency. In Ni-Zn, purifying liquid raised removal by 10%, and the penetrant boosted it by 300%. In Cu-Zn, varnish and purifying liquid increased removal by 200%, while the penetrant caused a 400% drop. These results underscore the importance of accounting for organic additives when designing electroflotation systems, as they can significantly alter performance.
1 I.V. Butorina, M.V. Butorina, Chernye Metally 1 (2021) 43.
2 S. Meng, S.Wen, G. Han, X. Wang, Q. Feng, Water 14(5) (2022) 726. https://doi.org/10.3390/w14050726
3 A. Abrashov, N. Grigoryan, Y. Korshak, T.Vagramyan, O. Grafov, Y. Mezhuev, Metals 11(11) (2021) 1718. https://doi.org/10.3390/met11111718
4 Ya.O. Mezhuev, Yu.V. Korshak, T.A. Vagramyan, A.A. Abrashov, A.I. Piskareva, G.A. Yur'eva, M.I. Shtil'man, International Polymer Science & Technology 41(4) (2014) 53. https://doi.org/10.1177/0307174X1404100409
5 E.G. Vinokurov, V.D. Skopintsev, Kh.A. Nevmyatullina, А.V. Morgunov, Khimicheskaya promyshlennost segodnya 10 (2016) 18.
6 E.N. Kuzin, N.E. Kruchinina, P.I. Chernyshev, N.S. Vizen, Inorganic Materials 56 (2020) 507. https://doi.org/10.1134/S002016852005009X
7 E. Bibaj, K. Lysigaki, J.W. Nolan, M. Seyedsalehi, E.A. Deliyanni, A.C. Mitropoulos, G.Z. Kyzas, Int. J. Environ. Sci. Technol. 16 (2019) 667. https://doi.org/10.1007/s13762-018-1676-0
8 R.R.V. Hemavathy, P.S. Kumar, S. Suganya, S.J.Swetha, Bioresour. Technol. 281 (2019) 5. https://doi.org/10.1016/j.biortech.2019.02.070
9 Е.N. Kuzin, Yu.M. Averina, А.Yu. Kurbatov, P.А. Sakharov, Tsvetnye metally 10 (2019) 91. http://dx.doi.org/10.17580/tsm.2019.10.15
10 Y. Li, X. Zeng, Y. Liu, Sh. Yan, Z. Hu, Y. Ni, Separation and Purification Technology 31(1) (2003) 91. https://doi.org/10.1016/S1383-5866(02)00162-4
11 H. Celebi, Journal of the Chemical Society of Pakistan 43(2) (2021) 114. https://doi.org/10.52568/000565
12 A. Ali Redha, Arab Journal of Basic and Applied Sciences 27(1) (2020) 183. https://doi.org/10.1080/25765299.2020.1756177
13 R. Shahrokhi-Shahraki, C. Benally, M.G. El-Din, J. Park, Chemosphere 264(1) (2021) 128. https://doi.org/10.1016/j.chemosphere.2020.128455
14 Y. Huang, D. Wu, X. Wang, W. Huang, D. Lawless, X. Feng, Separation and Purification Technol. 158 (2016) 124. https://doi.org/10.1016/j.seppur.2015.12.008
15 M. Vemula, V.B. Ambavaram, G.R. Kalluru, G. Madhavi, T.N.V.K.V. Prasad, Research Journal of Recent Sciences 2(1) (2013) 71.
16 А.Yu. Kurbatov, А.B. Fadeev, Yu.М. Averina, М.А. Vetrova, Tsvetnye metally 10 (2021) 55.
17 J.M. Averina, G.E. Kaliakina, D.Y. Zhukov, A.Y. Kurbatov, V.S. Shumova, 19th International Multidisciplinary Scientific Geoconference 19 (2019) 145. https://doi.org/10.5593/sgem2019/3.1/S12.019
18 Z. Guojun, Chem Eng Equip. 7 (2022) 264.
19 Z.H. Zhang, Leather Manuf Environ Technol. 3(11) (2022) 94.
20 G.J. Zeng, Chem Eng Equip. 7 (2022) 264.
21 Y. Yang, X. Jie, Journal of Environmental and Occupational Medicine 40(11) (2023) 1347. https://dx.doi.org/10.11836/JEOM23068
22 W. Xiwei, W. Qiuhong, Yu. Lihua, China Agric Bull 33(19) (2017) 86.
23 S. Das, K.W. Sultana, A.R. Ndhlala, M. Mondal, I. Chandra, Environ Health Insights 17 (2023) 786. https://doi.org/10.1177/11786302231201259
24 S.S. Vinogradov, 2nd ed., revised and expanded - Мoscow: Globus (2002) 352 p.
25 Q. Chen, Y. Yao, X. Li, J. Lu, J. Zhou, Z. Huang, Journal of water process engineering 26 (2018) 289. https://doi.org/10.1016/j.jwpe.2018.11.003
26 Yu.М. Averina, G.Е. Kalyakina, V.V. Menshikov, Yu.I. Kapustin, V.S. Boldyrev, Vestnik Moskovskogo gosudarstvennogo tekhnicheskogo universiteta im. N.E.Baumana. Ser. «Estestvennyenauki» 3(84) (2019) 70. http://dx.doi.org/10.18698/1812-3368-2019-3-70-80
27 Е.N. Kuzin, N.Е. Kruchinina, Tsvetnye metally 10 (2016) 8. http://dx.doi.org/10.17580/tsm.2016.10.01
28 M. Ince, I.O. Kaplan, A critical review, International Journal of Pure and Applied Sciences 2 (2017) 10. http://dx.doi.org/10.29132/ijpas.372335
29 N.A.A. Qasem, R.H. Mohammed, D.U. Lawal, Clean Water 4 (2021) 36. https://doi.org/10.1038/s41545-021-00127-0
30 Z. Ying, Z. Aimin, L. Zhiqiang, Carbon Lett. 34 (2024) 177. https://doi.org/10.1007/s42823-023-00660-7
31 A. Bashir, L.A. Malik, S. Ahad, T. Manzoor, M.A. Bhat, Environ. Chem. Lett. 17 (2019) 729. https://doi.org/10.1080/25765299.2020.1756177
32 R. Shahrokhi-Shahraki, C. Benally, M.G. El-Din, J. Park, Chemosphere 264(1) (2021) 128. https://doi.org/10.1016/j.chemosphere.2020.128455
33 L.B. Chaudhari, Z.V.P. Murthy, J Hazard Mater 180(1-3) (2010) 305. https://doi.org/10.1016/j.jhazmat.2010.04.032
34 C.V. Gherasim, P. Mikulášek, Desalination 343 (2014) 67. https://doi.org/10.1016/j.desal.2013.11.012
35 A.V. Kolesnikov, P. Aung, T.V. Davydkova, V.A. Kolesnikov, Nonferrous Metals 1 (2021) 3. https://doi.org/10.17580/nfm.2021.01.01
36 A.V. Kolesnikova, M.G. Achkasova, G.I. Kandelakia, Russian Journal of Applied Chemistry 91 (2018) 915.
37 V.A. Kolesnikov, V.I. Il’in, V.A. Brodskiy, A.V. Kolesnikov, Theoretical Foundations of Chemical Engineering 51 (2017) 369. https://doi.org/10.1134/S1070427218060058
38 Е.N. Kuzin, Chernye metally 10 (2022) 79. https://doi.org/10.17580/chm.2022.10.13
39 V.А. Kolesnikov, L.А. Kryuchkova, V.I. Ilyin, А.V. Kolesnikov, Galvanotekhnika i obrabotka poverkhnosti 23 (2015) 51.
40 P. Aung, Т.А. Hein, А.V. Kolesnikov, Galvanotekhnika i obrabotka poverkhnosti 28 (2020) 38. https://doi.org/10.47188/0869-5326 2020 28 4 38
H.T. Aung, A.V. Kolesnikov, Yu.M. Averina, V.V. Chelnokov, F.M. Chyragov, Effect of background electrolytes and pH on electroflotation efficiency for heavy metal removal, UNEC J. Eng. Appl. Sci. 5(1) (2025) 115-123. https://doi.org/10.61640/ujeas.2025.0512
Anyone you share the following link with will be able to read this content:

This article is licensed under the Creative Commons Attribution ( CC BY 4.0 ) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
I.V. Butorina, M.V. Butorina, Chernye Metally 1 (2021) 43.
S. Meng, S.Wen, G. Han, X. Wang, Q. Feng, Water 14(5) (2022) 726. https://doi.org/10.3390/w14050726
A. Abrashov, N. Grigoryan, Y. Korshak, T.Vagramyan, O. Grafov, Y. Mezhuev, Metals 11(11) (2021) 1718. https://doi.org/10.3390/met11111718
Ya.O. Mezhuev, Yu.V. Korshak, T.A. Vagramyan, A.A. Abrashov, A.I. Piskareva, G.A. Yur'eva, M.I. Shtil'man, International Polymer Science & Technology 41(4) (2014) 53. https://doi.org/10.1177/0307174X1404100409
E.G. Vinokurov, V.D. Skopintsev, Kh.A. Nevmyatullina, А.V. Morgunov, Khimicheskaya promyshlennost segodnya 10 (2016) 18.
E.N. Kuzin, N.E. Kruchinina, P.I. Chernyshev, N.S. Vizen, Inorganic Materials 56 (2020) 507. https://doi.org/10.1134/S002016852005009X
E. Bibaj, K. Lysigaki, J.W. Nolan, M. Seyedsalehi, E.A. Deliyanni, A.C. Mitropoulos, G.Z. Kyzas, Int. J. Environ. Sci. Technol. 16 (2019) 667. https://doi.org/10.1007/s13762-018-1676-0
R.R.V. Hemavathy, P.S. Kumar, S. Suganya, S.J.Swetha, Bioresour. Technol. 281 (2019) 5. https://doi.org/10.1016/j.biortech.2019.02.070
Е.N. Kuzin, Yu.M. Averina, А.Yu. Kurbatov, P.А. Sakharov, Tsvetnye metally 10 (2019) 91. http://dx.doi.org/10.17580/tsm.2019.10.15
Y. Li, X. Zeng, Y. Liu, Sh. Yan, Z. Hu, Y. Ni, Separation and Purification Technology 31(1) (2003) 91. https://doi.org/10.1016/S1383-5866(02)00162-4
H. Celebi, Journal of the Chemical Society of Pakistan 43(2) (2021) 114. https://doi.org/10.52568/000565
A. Ali Redha, Arab Journal of Basic and Applied Sciences 27(1) (2020) 183. https://doi.org/10.1080/25765299.2020.1756177
R. Shahrokhi-Shahraki, C. Benally, M.G. El-Din, J. Park, Chemosphere 264(1) (2021) 128. https://doi.org/10.1016/j.chemosphere.2020.128455
Y. Huang, D. Wu, X. Wang, W. Huang, D. Lawless, X. Feng, Separation and Purification Technol. 158 (2016) 124. https://doi.org/10.1016/j.seppur.2015.12.008
M. Vemula, V.B. Ambavaram, G.R. Kalluru, G. Madhavi, T.N.V.K.V. Prasad, Research Journal of Recent Sciences 2(1) (2013) 71.
А.Yu. Kurbatov, А.B. Fadeev, Yu.М. Averina, М.А. Vetrova, Tsvetnye metally 10 (2021) 55.
J.M. Averina, G.E. Kaliakina, D.Y. Zhukov, A.Y. Kurbatov, V.S. Shumova, 19th International Multidisciplinary Scientific Geoconference 19 (2019) 145. https://doi.org/10.5593/sgem2019/3.1/S12.019
Z. Guojun, Chem Eng Equip. 7 (2022) 264.
Z.H. Zhang, Leather Manuf Environ Technol. 3(11) (2022) 94.
G.J. Zeng, Chem Eng Equip. 7 (2022) 264.
Y. Yang, X. Jie, Journal of Environmental and Occupational Medicine 40(11) (2023) 1347. https://dx.doi.org/10.11836/JEOM23068
W. Xiwei, W. Qiuhong, Yu. Lihua, China Agric Bull 33(19) (2017) 86.
S. Das, K.W. Sultana, A.R. Ndhlala, M. Mondal, I. Chandra, Environ Health Insights 17 (2023) 786. https://doi.org/10.1177/11786302231201259
S.S. Vinogradov, 2nd ed., revised and expanded - Мoscow: Globus (2002) 352 p.
Q. Chen, Y. Yao, X. Li, J. Lu, J. Zhou, Z. Huang, Journal of water process engineering 26 (2018) 289. https://doi.org/10.1016/j.jwpe.2018.11.003
Yu.М. Averina, G.Е. Kalyakina, V.V. Menshikov, Yu.I. Kapustin, V.S. Boldyrev, Vestnik Moskovskogo gosudarstvennogo tekhnicheskogo universiteta im. N.E.Baumana. Ser. «Estestvennyenauki» 3(84) (2019) 70. http://dx.doi.org/10.18698/1812-3368-2019-3-70-80
Е.N. Kuzin, N.Е. Kruchinina, Tsvetnye metally 10 (2016) 8. http://dx.doi.org/10.17580/tsm.2016.10.01
M. Ince, I.O. Kaplan, A critical review, International Journal of Pure and Applied Sciences 2 (2017) 10. http://dx.doi.org/10.29132/ijpas.372335
N.A.A. Qasem, R.H. Mohammed, D.U. Lawal, Clean Water 4 (2021) 36. https://doi.org/10.1038/s41545-021-00127-0
Z. Ying, Z. Aimin, L. Zhiqiang, Carbon Lett. 34 (2024) 177. https://doi.org/10.1007/s42823-023-00660-7
A. Bashir, L.A. Malik, S. Ahad, T. Manzoor, M.A. Bhat, Environ. Chem. Lett. 17 (2019) 729. https://doi.org/10.1080/25765299.2020.1756177
R. Shahrokhi-Shahraki, C. Benally, M.G. El-Din, J. Park, Chemosphere 264(1) (2021) 128. https://doi.org/10.1016/j.chemosphere.2020.128455
L.B. Chaudhari, Z.V.P. Murthy, J Hazard Mater 180(1-3) (2010) 305. https://doi.org/10.1016/j.jhazmat.2010.04.032
C.V. Gherasim, P. Mikulášek, Desalination 343 (2014) 67. https://doi.org/10.1016/j.desal.2013.11.012
A.V. Kolesnikov, P. Aung, T.V. Davydkova, V.A. Kolesnikov, Nonferrous Metals 1 (2021) 3. https://doi.org/10.17580/nfm.2021.01.01
A.V. Kolesnikova, M.G. Achkasova, G.I. Kandelakia, Russian Journal of Applied Chemistry 91 (2018) 915.
V.A. Kolesnikov, V.I. Il’in, V.A. Brodskiy, A.V. Kolesnikov, Theoretical Foundations of Chemical Engineering 51 (2017) 369. https://doi.org/10.1134/S1070427218060058
Е.N. Kuzin, Chernye metally 10 (2022) 79. https://doi.org/10.17580/chm.2022.10.13
V.А. Kolesnikov, L.А. Kryuchkova, V.I. Ilyin, А.V. Kolesnikov, Galvanotekhnika i obrabotka poverkhnosti 23 (2015) 51.
P. Aung, Т.А. Hein, А.V. Kolesnikov, Galvanotekhnika i obrabotka poverkhnosti 28 (2020) 38. https://doi.org/10.47188/0869-5326 2020 28 4 38