UNEC Journal of Engineering and Applied Sciences Volume 5, No 1, pages 91-95 (2025) Cite this article, 848 https://doi.org/10.61640/ujeas.2025.0510
As we know, one of the major energy sources is crude oil. Crude oil is refined into transportation fuels such as gasoline, diesel, kerosene, and jet fuel. [1]. The combustion of fuels causes many toxic gases, such as nitrogen-sulfur oxide and hydrogen sulfide. Growing attention to air pollution has been laid down to limit the sulfur content in fuel. The sulfur content in the specification of gasoline and diesel fuels can be 10 parts per million (ppm) [2]. The traditional industry hydro-cleaning method for removing nitrogen, sulfur, and aromatic compounds in fuels requires high temperatures, pressure, and costly catalysts [3,4]. On the other hand, this process reduces the octane (or cetane) number of fuels. Moreover, the low reaction activity of thiophene, dibenzothiophene, and their derivatives cannot be fully separated from fuel [5,6]. Therefore, the development of economical and ecological efficient cleaning methods is very important.
Hence, alternative methods such as extraction, oxidation, biological separation, adsorption, and others have been studied [7-11]. Among them, the oxidation desulfurization process (ODS) needs no hydrogen consumption and mild reaction conditions [12,13]. In this process, sulfur components oxidized to sulfoxides or sulfones. Generally, H2O2, NO/NO2, O3, KMnO4, and molecular oxygen were used as oxidative agents [14-16]. The most important factor in ODS is the applying of selective and eco-friendly extractive solvents.
Deep eutectic solvents (DESs), also known as green solvents, were investigated at the beginning of the 21st century [17]. DESs present low volatility, low vapor, and high thermal stability. They are non-toxic solvents [18,19]. DESs are applied in different fields, such as absorption of acidic gases, extraction of bioactive compounds, solvent-catalyst in organic synthesis and removal of glycerol from biodiesel [20].
In this work, we report a novel series of glycerin-based deep eutectic solvents used as extractive solvents in the ODS process. Ammonium chloride, triethylammonium acetate, and choline chloride were chosen as hydrogen bond acceptors of DESs. Hydrogen peroxide was used as an oxidative agent in the purification of model diesel fuels. Thiophene and dibenzothiophene were added to the model diesel as sulfur components. As result we can note that extraction efficiency of investigated DESs was 81-100% in the ODS processes for thiophene and dibenzothiophene. The separation efficiency was confirmed by NMR methods.
2.1 Materials
Glycerol, ammonium chloride, n-decane, hexadecane, thiophene (98%), dibenzothiophene (DBT, 99%) and choline chloride were obtained by Merck (Germany). Triethylammonium acetate [TEAH]+[AcO]- was synthesized in the laboratory and the structure was confirmed by NMR.
2.2 Preparation of DESs and model diesel fuel
The DESs were prepared via a single-step synthesis process. Ammonium chloride, choline chloride, and triethylammonium acetate were chosen as HBA, glycerol was taken as the HBD of DESs. Molar ratios of HBA/ HBD were taken 1:6. The mixing process continued until a clear homogeneous liquid appeared.
The model diesel fuels containing thiophene (5%) and dibenzothiophene (DBT, 2%) were prepared by dissolving in n-decane and hexadecane. The components of the model fuel were n-decane and hexadecane with the volume ratio of 1:1.
2.3 Oxidative desulfurization process
The ODS processes were carried out at 60° and 90°C temperatures in 1- and 3-hours of mixing times. The DES, 30 wt% H2O2, and model fuel mixture of were added to a 100 mL-bottomed round flask. The experiments were conducted with the help of a heating magnetic stirrer. The molar ratios of DES/model fuel/H2O2 were taken 1:1:2. A white solid was observed in the DBT oxidative desulfurization system. The following equations define the desulfurization rate.
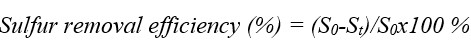
where S0 -total sulfur concentration, St- residual sulfur concentration after t h.
2.4 NMR analysis
NMR experiments have been performed on a BRUKER FT NMR spectrometer (UltraShieldTM Magnet) AVANCE 300 (300.130 MHz for 1H and 75.468 MHz for 13C) with a BVT 3200 variable temperature unit in 5 mm sample tubes using Bruker Standard software (TopSpin 3.1). The 1H chemical shifts were referenced to internal tetramethylsilane (TMS); the experimental parameters for 1H: digital resolution = 0.23 Hz, SWH = 7530 Hz, TD = 32 K, SI = 16 K, 900 pulse-length = 10 μs, PL1 = 3 dB, ns-= 1, ds= 0, d1 =1 s; for 13C: digital resolution = 0.27 Hz, SWH = 17985 Hz, TD = 64 K, SI = 32 K, 900 pulse-length = 9 μs, PL1 = 1.5 dB, ns= 100, ds= 2, d1= 3 s. NMR-grade CDCl3 was used for the analysis of model fuel blends.
Oxidation desulfurization process
Thiophene and DBT were selected as sulfuric components to explore the extraction capacity of DESs in the oxidation desulfurization process. The results are shown in table 1-5. In all experiments, the molar ratio of DES/model fuel was taken as 1:1. The volume ratio of the oxidative agent H2O2 to the DES/model fuel system was used 2:1:1. ODS processes were carried out at 60° and 90°C. Liquid-liquid extraction times were chosen as 1 and 3 hours.
Firstly, NH4Cl/6Glycerol, [TEAH]+[AcO]-/6Glycerol, and ChCl/6Glycerol were used as the extractive solvents in ODS for the separation of thiophene from model diesel fuel. As can be seen from Table 1, the optimal efficiency of thiophene was 63 % by ChCl/6Glycerol in three hours at 60°C. Analysis of each sample by NMR showed NH4Cl/6Glycerol and ChCl/6Glycerol completely removed thiophene from the model diesel fuel at 1 hour and 90°C temperature. [TEAH]+[AcO]-/6Glycerol performed high-extraction efficiency in 3 hours of mixing times.
The above-indicated DESs were also used as the extractive solvents in ODS for the separation of DBT from model diesel fuel (table 2, figure 1). From table 2, it is obvious that the high removal efficiency of DBT was observed in three hours at 60°C temperature by NH4Cl/6Glycerol. The desulfurization rate was 77 %. However, the higher removal efficiency of DBT was 95% at 90°C temperature by NH4Cl/6Glycerol in 3 hours. Beside it, the higher extraction capacity of ChCl/6Glycerol is 84% in 1 hour of mixing time. Under the same conditions, [TEAH]+[AcO]-/6Glycerol showed a higher result in 3 hours.
DES phase was separated from model fuel by a simple decanting. Then a white solid phase is filtered from the DES phase.
The separation of thiophene/DBT mixtures by DESs was investigated at 90°C for 1 and 3 hours (table 3). Extraction at 3 hours showed better separation efficiencies. The purification effect of ChCl/6Glycerol is 81%. The maximum extraction of thiophene/DBT by NH4Cl/6Glycerol and [TEAH]+[AcO]-/6Glycerol was 73 and 63%, respectively.
Regeneration of DESs
Regenerating DESs is an essential factor in their large-scale applications. To obtain the reuse of DESs in ODS, we used diethyl ether for the purification process. The volume ratios of DES/diethyl ether were 1:1. The purification process was carried out in 3 hours at room temperature with the help of a magnetic stirrer. The results show that regenerated DESs extracted thiophene and DBT with 80-85% purification in a single stage. All analyses were confirmed by 1H NMR.
In this study, a new type of green solvent called deep eutectic solvents was synthesized and investigated in the oxidation desulfurization process. Glycerol is a hydrogen bond donor, ammonium chloride, [TEAH+][AcO-] chloride, and choline chloride are hydrogen bond acceptors of DESs. Thiophene (5%) and dibenzothiophene (2%) were added as sulfuric components of model diesel fuel. Oxidation desulfurization processes were carried out in 1 and 3 hours at 60° and 90°C temperatures by taking 30% H2O2. Thiophene was completely (100%) removed from the model diesel fuel in 3 hours by [TEAH]+[AcO]-/6Glycerol at 90°C temperature. NH4Cl/6Glycerol and ChCl/6Glycerol showed similar results at the same temperature in 1 hour. The increasing temperature made better extraction for the sulfuric components in ODS processes. The higher efficiency of DBT (81%) was shown in 3 hours at 90°C temperature by ChCl/6Glycerol.
1 K.E. Seiferlein Annual energy review 2007 (2008). https://doi.org/10.2172/1212314
2 OECD/IEA, Energy and air pollution: world energy outlook special report (2016).
3 I. Mochida, K.H. Choi, Journal of the Japan Petroleum Institute 47(3) (200) 145. http://dx.doi.org/10.1627/jpi.47.145
4 M.J. Girgis, B.C. Gates, Industrial & Engineering Chemistry Research 30(9) (1991) 2021. https://doi.org/10.1021/ie00057a001
5 W. Liu, T. Li, G. Yu, J. Wang, Z. Zhou, Fuel 265 (2020) 116967. http://dx.doi.org/10.1016/j.fuel.2019.116967
6 C.F. Mao, R.X. Zhao, X.P. Li, Fuel 189 (2017) 400. https://doi.org/10.1016/j.fuel.2016.10.113
7 A. Koriakin, K.M. Ponvel, C.H. Lee, Chemical Engineering Journal 162(2) (2010) 649. http://dx.doi.org/10.1016/j.cej.2010.06.014
8 K. Kirimura, T. Furuya, Y. Nishii, Y. Ishii, K. Kino, S. Usami, Journal of bioscience and bioengineering 91(3) (2001) 262. https://doi.org/10.1016/S1389-1723(01)80131-6
9 J.J. Li, H. Xiao, X.D. Tang, M. Zhou, Energy & Fuels 30(7) (2016) 5411. http://dx.doi.org/10.1021/acs.energyfuels.6b00471
10 C. Li, D. Li, S. Zou, Z. Li, J. Yin, A. Wang, Y. Cui, Z. Yao, Q. Zhao, Green Chemistry 15(10) (2013) 2793. https://doi.org/10.1039/C3GC41067F
11 J. Yin, J. Wang, Z. Li, D. Li, G. Yang, Y. Cui, A. Wang, C. Li, Green Chemistry 17(9) (2015) 4552. https://doi.org/10.1039/C5GC00709G
12 J. Xiong, W. Zhu, W. Ding, L. Yang, Y. Chao, H. Li, F. Zhu, H. Li, Industrial & Engineering Chemistry Research 53(51) (2014) 19895. https://doi.org/10.1021/ie503322a
13 W. Zhu, P. Wu, L. Yang, Y. Chang, Y. Chao, H. Li, Y. Jiang, W. Jiang, S. Xun, Chemical engineering journal 229 (2013) 250. https://doi.org/10.1016/j.cej.2013.05.115
14 C.F. Mao, R.X. Zhao, X.P. Li, RSC advances 7(67) (2017) 42590. https://doi.org/10.1039/C7RA05687G
15 M.H. Ibrahim, M. Hayyan, M.A. Hashim, A. Hayyan, Renewable and Sustainable Energy Reviews 76 (2017) 1534. http://dx.doi.org/10.1016/j.rser.2016.11.194
16 V.C. Srivastava, Rsc Advances 2(3) (2012) 759. https://doi.org/10.1039/C1RA00309G
17 Á. Santana-Mayor, R. Rodríguez-Ramos, A.V. Herrera-Herrera, B. Socas-Rodríguez, M.Á. Rodríguez-Delgado, TrAC Trends in Analytical Chemistry 134 (2021) 116108. http://dx.doi.org/10.1016/j.trac.2020.116108
18 W. Chen, Z. Xue, J. Wang, J. Jiang, X. Zhao, T. Mu, Acta Phys. Chim. Sin. 34(8) (2018) 904. https://doi.org/10.3866/PKU.WHXB201712281
19 L. Lomba, M.P. Ribate, E. Sangüesa, J, Concha, Mª. Garralaga, D. Errazquin, C.B. García, B. Giner, Applied Sciences 11(21) (2021) 10061. https://doi.org/10.3390/app112110061
20 Y.C. Hou, C.F. Yao, W.Z. Wu, Acta Phys.-Chim. Sin. 34(8) (2018) 873. https://doi.org/10.3866/PKU.WHXB201802062
I.G. Mammedov, S.E. Niftullayeva, Y.V. Mamedova, Eco-friendly oxidative desulfurization of model diesel fuel using glycerol-based deep eutectic solvents, UNEC J. Eng. Appl. Sci. 5(1) (2025) 91-95. https://doi.org/10.61640/ujeas.2025.0510
Anyone you share the following link with will be able to read this content:

This article is licensed under the Creative Commons Attribution ( CC BY 4.0 ) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
K.E. Seiferlein Annual energy review 2007 (2008). https://doi.org/10.2172/1212314
OECD/IEA, Energy and air pollution: world energy outlook special report (2016).
I. Mochida, K.H. Choi, Journal of the Japan Petroleum Institute 47(3) (200) 145. http://dx.doi.org/10.1627/jpi.47.145
M.J. Girgis, B.C. Gates, Industrial & Engineering Chemistry Research 30(9) (1991) 2021. https://doi.org/10.1021/ie00057a001
W. Liu, T. Li, G. Yu, J. Wang, Z. Zhou, Fuel 265 (2020) 116967. http://dx.doi.org/10.1016/j.fuel.2019.116967
C.F. Mao, R.X. Zhao, X.P. Li, Fuel 189 (2017) 400. https://doi.org/10.1016/j.fuel.2016.10.113
A. Koriakin, K.M. Ponvel, C.H. Lee, Chemical Engineering Journal 162(2) (2010) 649. http://dx.doi.org/10.1016/j.cej.2010.06.014
K. Kirimura, T. Furuya, Y. Nishii, Y. Ishii, K. Kino, S. Usami, Journal of bioscience and bioengineering 91(3) (2001) 262. https://doi.org/10.1016/S1389-1723(01)80131-6
J.J. Li, H. Xiao, X.D. Tang, M. Zhou, Energy & Fuels 30(7) (2016) 5411. http://dx.doi.org/10.1021/acs.energyfuels.6b00471
C. Li, D. Li, S. Zou, Z. Li, J. Yin, A. Wang, Y. Cui, Z. Yao, Q. Zhao, Green Chemistry 15(10) (2013) 2793. https://doi.org/10.1039/C3GC41067F
J. Yin, J. Wang, Z. Li, D. Li, G. Yang, Y. Cui, A. Wang, C. Li, Green Chemistry 17(9) (2015) 4552. https://doi.org/10.1039/C5GC00709G
J. Xiong, W. Zhu, W. Ding, L. Yang, Y. Chao, H. Li, F. Zhu, H. Li, Industrial & Engineering Chemistry Research 53(51) (2014) 19895. https://doi.org/10.1021/ie503322a
W. Zhu, P. Wu, L. Yang, Y. Chang, Y. Chao, H. Li, Y. Jiang, W. Jiang, S. Xun, Chemical engineering journal 229 (2013) 250. https://doi.org/10.1016/j.cej.2013.05.115
C.F. Mao, R.X. Zhao, X.P. Li, RSC advances 7(67) (2017) 42590. https://doi.org/10.1039/C7RA05687G
M.H. Ibrahim, M. Hayyan, M.A. Hashim, A. Hayyan, Renewable and Sustainable Energy Reviews 76 (2017) 1534. http://dx.doi.org/10.1016/j.rser.2016.11.194
V.C. Srivastava, Rsc Advances 2(3) (2012) 759. https://doi.org/10.1039/C1RA00309G
Á. Santana-Mayor, R. Rodríguez-Ramos, A.V. Herrera-Herrera, B. Socas-Rodríguez, M.Á. Rodríguez-Delgado, TrAC Trends in Analytical Chemistry 134 (2021) 116108. http://dx.doi.org/10.1016/j.trac.2020.116108
W. Chen, Z. Xue, J. Wang, J. Jiang, X. Zhao, T. Mu, Acta Phys. Chim. Sin. 34(8) (2018) 904. https://doi.org/10.3866/PKU.WHXB201712281
L. Lomba, M.P. Ribate, E. Sangüesa, J, Concha, Mª. Garralaga, D. Errazquin, C.B. García, B. Giner, Applied Sciences 11(21) (2021) 10061. https://doi.org/10.3390/app112110061
Y.C. Hou, C.F. Yao, W.Z. Wu, Acta Phys.-Chim. Sin. 34(8) (2018) 873. https://doi.org/10.3866/PKU.WHXB201802062