UNEC Journal of Engineering and Applied Sciences Volume 4, No 2, pages 12-19 (2024) Cite this article, 1682 https://doi.org/10.61640/ujeas.2024.1202
In recent times, nanomaterials have attracted the attention of the scientific community due to their unique properties and various technological applications. The different properties of nanoscale materials compared to macroscale materials often result from size limitations, the dominance of interfacial processes, and quantum effects. These new and distinctive properties of nanostructured materials lead to improvements in catalyst efficiency, tunable photoactivity, power enhancement, and many other intriguing characteristics [1]. Silver nanoparticles (AgNPs) of various shapes are widely used in biomedical imaging as optical labels and substrates for surface-enhanced Raman spectroscopy due to their optical properties, particularly localized surface plasmon resonance. The characteristics of AgNPs, which vary significantly according to shape and size, are crucial for these applications. Many studies demonstrate that the surface plasmon resonance (SPR) of Ag nanoparticles fundamentally depends on the number and position of peaks, size, and dielectric environment of the metal nanoparticles [2]. Metal nanoparticles are characterized by high conductivity, a large surface-to-volume ratio, and plasmonic properties [3- 4]. SPR properties enable the amplification and manipulation of light at the nanoscale level, thereby enhancing the sensitivity and resolution of optical devices. Among various metal nanoparticles, silver (Ag) is widely studied due to its high permeability, excellent light absorption, high sensitivity, resolution, antibacterial activity, and chemical stability [5- 7].
Diphenylcarbazide (DPC) is a widely used reagent in analytical chemistry, primarily due to its ability to form colored complexes with various metal ions [8- 11]. With the chemical formula C₁₂H₁₁N₃O, diphenylcarbazide is a derivative of carbazide in which two phenyl groups are substituted at the nitrogen atom. This organic compound is utilized as an indicator for iron titration and in the colorimetric determination of Cr (VI). DPC contains two amino groups that can interact with the surface of nanoparticles through hydrogen bonding. In this work, we utilize DPC as a selective ligand to modify silver nanoparticles for the determination of various metals.
The purpose of this article is to study the synthesis and structure of new complexes based on silver nanoparticles, diphenylcarbazide reagent, and cetyltrimethylammonium bromide (CTAB), which can be effective in the determination of many elements using photometric and other methods in analytical chemistry.
1.1.Materials
Silver nitrate (AgNO3, PLC 141459, 98% chemically pure), soluble starch ((C6H10O5)n, PLC 121096, 98% chemically pure), β-D glucose (C6H12O6, CAS No.50-99-7, 98% chemically pure); sodium hydroxide (NaOH, PLC 141687, 98% chemically pure), cetyltrimethylammonium bromide (CTAB, AB 117004, 98% chemically pure), ethanol (C2H5OH, CAS No.64-17-5, 95% chemically pure.), diphenyl carbazide DPC (CAS140-22-7), 98% chemically pure) were used as received.
1.2.Synthesis of Ag Nanoparticles
The synthesis and stabilization of Ag nanoparticles were carried out in the following steps: First, 150 mL of a 1% starch solution was added to 100 mL of a 0.01 M AgNO₃ solution. Next, 100 mL of a 0.07 M sodium hydroxide solution was mixed with 100 mL of a 0.2 M glucose solution. This prepared NaOH and glucose solution was then added to the AgNO₃ and starch mixture and stirred for 30 minutes. Immediately after this process, the solution turned dark brown, indicating the formation of a colloidal solution of Ag nanoparticles. Then, to clean the Ag nanoparticles from extraneous and unreacted ions, the nanoparticles are separated in an R 5430 Eppendorf ultracentrifuge at 12,000 rpm for 30 minutes and washed several times with in equal volume ratios of water and ethanol. This process was performed under ambient conditions.In this synthesis process, starch serves as both a reducing agent and a stabilizer, while NaOH acts as a reaction accelerator. Glucose also functions as a reducing agent.
1.3. Synthesis of Ag+DPC+CTAB-Based Complex
In this work, the synthesis of new complexes based on silver nanoparticles, diphenylcarbazide (DPC) reagent, and cetyltrimethylammonium bromide (CTAB) was carried out. First, a 10⁻³ M concentration solution of the DPC reagent was prepared in a 1:1 water-alcohol mixture. To this, 10 mL of the pre-prepared 0.01 M Ag nanoparticles solution was added to 50 mL of the 10⁻³ M DPC reagent and stirred on a magnetic stirrer for 2 hours. The color of the resulting solution changed from dark red to red, indicating the formation of a binary complex. For the synthesis of the ternary complex, an additional 10 mL of the 0.01 M Ag nanoparticles solution was added to 50 mL of the 10⁻³ M DPC reagent and stirred for an additional 2 hours. After this, 5 mL of a 0.5% (0.01 M) CTAB solution was added, and stirring continued to obtain the ternary complex. The obtained binary and ternary complexes are dried by heating in a muffle furnace at 120 degrees for 3 hours to remove any residual solvent and moisture.CTAB acts as a stabilizer in the complex formation process, ensuring the stability of the silver nanoparticles with the reactants.
Research Methods
Ultraviolet spectra of the samples were obtained using a SPECORD 210 PLUS spectrophotometer (Analytic Jena, Germany) at wavelengths ranging from 200 to 900 nm at room temperature. The infrared (IR) spectra of the samples were measured with a Varian 640 infrared spectrometer at wavelengths of 400 to 4000 cm⁻¹, also at room temperature. X-ray analysis of the reagent and complexes was conducted at room temperature using a Rigaku Mini flex 600 diffractometer (Japan).
Figure 1 shows the XRD diffractograms of the DFC reagent (a), DPC+Ag binary complex (b), and DPC+Ag+CTAB ternary complex (c). The crystallite sizes of the reagent, binary, and ternary complexes were calculated using the Debye-Scherrer formula (1) :
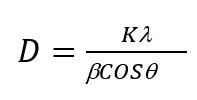 (1)
(1)
where K is a unitless constant (K=0.94), λ is the wavelength of the incident X-ray (λ=1.5406 Å, Cu Kα), θ is the angle corresponding to the diffraction maximum, and β is the full width at half maximum (FWHM). According to the XRD diffractograms, the average size of the crystallites in the DPC reagent is 16.06 nm, while in the DPC+Ag binary complex- 11.42 nm and the DPC+Ag+CTABr ternary complex, it is 13.65 nm. In the XRD diffractogram of the DPC+Ag binary complex, peaks are observed at 2θ angles of 38.21°, 44.36°, 64.47°, 77.43°, and 81.54°. For the DPC+Ag+CTABr ternary complex, maxima appear at 2θ angles of 31.15°, 38.35°, 44.57°, 55.30°, 64.65°, 73.39°, 77.58°, and 81.77°.

Table 1. Data of DPC reagent, DPC+Ag double and DPC+Ag+CTAB triple complexes estimated by X-ray diffractometry method
IR Spectroscopy provides information about the characteristic vibrations of functional groups in compounds and helps analyze their molecular structure [11- 15]. Diphenylcarbazide, also known as diphenylcarbazone, has the chemical formula C₁₃H₁₁N₃O. The IR spectrum allows for the identification of functional groups such as aromatic rings, carbonyl groups, and carbazide moieties in diphenylcarbazide. Its structure features two phenyl rings connected by a carbazide group. The aromatic C-H bonds of the phenyl group appear as sharp peaks at around 3000-3100 cm⁻¹, corresponding to the stretching of C-H bonds in the aromatic system. The C=C bonds in the phenyl group produce peaks in the range of about 1500-1600 cm⁻¹, which are also sharp. Peaks around 1300-1400 cm⁻¹ correspond to the C-N bonds of the carbazide group, reflecting the interaction of the carbazide group with the phenyl rings. Changes in these peaks during the formation of the binary and ternary complexes indicate the establishment of coordination interactions between the carbazide group and silver nanoparticles (AgNPs). Peaks around 1650-1700 cm⁻¹ correspond to the carbonyl (C=O) bond of the carbazide group, indicating the presence of a carbonyl functional group. The intensity of this peak changes during the formation of the binary complex, suggesting coordination of the carbonyl group with the silver nanoparticles. Additionally, peaks related to the N-H groups appear around 1500-1600 cm⁻¹.
Figure 3 shows the UV spectra of the DFC reagent (1), DPC+Ag binary complex (2), and DPC+Ag+CTAB ternary complex (3). The UV spectra of silver nanoparticles indicate that the maximum intensity of their absorption bands varies between 400-450 nm, depending on the size of the nanoparticles [16-20]. As can be seen in figure 3 (a), the absorption band of the DFC reagent in the ultraviolet region is located in the range of 205-360 nm, with maximum peaks at 235 nm and 262 nm, corresponding to the π-π* transition of the C-C bond and the n-π* transition between C=N groups. Additional peaks are observed at wavelengths of 308 nm and 345 nm.
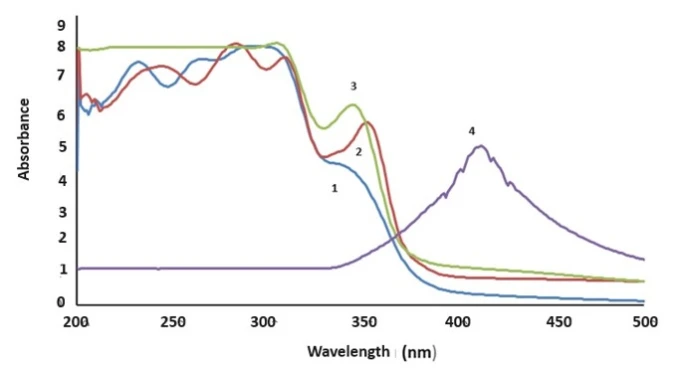
Figure 3. UV spectra of DPC reagent (1), DPC+Ag (2) and DPC+Ag+CTAB (3) complexes, and Ag (4) nanoparticles
Due to the surface plasmon resonance of the binary complex formed from the addition of 0.01 M silver nanoparticles to the reagent, the absorption curves shift to the right compared to the DPC reagent. This results in the emergence of new maximum peaks at wavelengths of 248 nm, 288 nm, 315 nm, and 355 nm. A bathochromic shift is observed during the formation of the binary complex, indicating a transition to a more acidic environment relative to the DPC reagent. The formation of the Ag+DPC nanocomposite is also accompanied by a noticeable color change from dark red to red. New peaks at longer wavelengths appear due to the aggregation of Ag nanoparticles. The Ag-R-CTAB ternary complex, formed by adding CTAB as a stabilizer to the complex created by the Ag nanoparticles with the DPC reagent, shows changes in the spectrum, with peaks in the absorption band observed at 305 nm and 348 nm. This alteration is attributed to quantum size effects. The formation of a complex with the DPC reagent, involving the coating of Ag nanoparticles with a surfactant, is explained by the generation of new peaks in the absorption band due to surface plasmon resonance and the color change from red to dark brown, which results from the aggregation of Ag nanoparticles. This aggregation also contributes to the bathochromic shift, moving the peak to longer wavelengths.
In figure 3, the displacement of the silver nanoparticles in the UV spectrum relative to the reactants and complexes is associated with the magnitude of the plasmon frequency. Thus, the plasma frequency of silver is greater than that of the binary and ternary complexes.
This study explores the synthesis and characterization of new complexes based on silver nanoparticles, diphenylcarbazide (DPC), and cetyltrimethylammonium bromide (CTAB). The novelty of this research lies in the combination of these components to form stable and spectroscopically distinctive complexes with potential applications in environmental sensing, catalysis, and the determination of metal ions. The observed changes in UV and IR spectra upon complex formation provide insights into the interactions between DPC and AgNPs, making these complexes promising candidates for use in analytical methods, such as photometric detection, and for environmental monitoring systems. The optical and spectral properties of the binary (DPC-AgNP) and ternary (DPC-AgNP-CTAB) complexes, as well as the binding interactions between DFC and AgNPs, were investigated using infrared (IR) and ultraviolet (UV) spectroscopy. The UV spectroscopy results indicate that the DPC reagent exhibits maximum peaks at wavelengths of 235 nm, 262 nm, 308 nm, and 345 nm. In the binary complex, surface plasmon resonance leads to the formation of new maximum peaks at 248 nm, 288 nm, 315 nm, and 355 nm. The ternary complex displays additional peaks at 305 nm and 348 nm, reflecting the structural changes and interactions within the complexes. The infrared spectroscopy results detail the spectral properties of DFC and its complexes, highlighting peaks associated with aromatic C-H and C=C bonds of the phenyl groups, as well as carbonyl (C=O) and C-N bonds of the carbazide group. The shifts in position and intensity of these peaks in the binary and ternary complex spectra indicate the coordination interactions between DFC and AgNPs. In conclusion, these studies underscore the importance of optimizing synthesis methods and exploring new applications to fully harness the potential of AgNPs across various fields of science and industry. By optimizing the synthesis conditions and studying the structural properties, we have created a foundation for the development of AgNP-based complexes that could be applied in various industrial and scientific fields, including sensor technologies, pollution detection, and as catalysts in chemical reactions. The structure and optical properties of the newly synthesized complexes have been determined, and their potential applications have been explored.
1 W.M. Shume, H.C. Ananda Murthy, E.A. Zereffa, Journal of Chemistry 2020 (2020). https://doi.org/10.1155/2020/5039479
2 M. Gao, L. Sun, Zh. Wang, Y. Zhao, Materials Science and Engineering 33(1) (2013) 397. https://doi.org/10.1016/j.msec.2012.09.005
3 K.V. Alex, P.T. Pavai, R. Rugmini, M.S. Prasad, ACS Omega 5(22) (2020) 13123. https://dx.doi.org/10.1021/acsomega.0c01136
4 S. Shariati, Gh. Khayatian, RSC Advances (11) (2021) 3295. https://doi.org/10.1039/D0RA08615K
5 A. Sanchez-Hachair, A. Hofmann, Comptes Rendus Chimie 21(9) (2018) 890. https://doi.org/10.1016/j.crci.2018.05.002endus
6 V. Kumar, D. Singh, S. Mohan, D. Bano, R.K. Gundampati, Journal of Photochemistry and Photobiology B: Biology 168 (2017) 67. https://doi.org/10.1016/j.jphotobiol.2017.01.022
7 M.A. Farooqi, S. Bae, S. Kim, S. Bae, Scientific Reports 14(1) (2024) 22922. https://doi.org/10.1038/s41598-024-723565
8 Z.A. Ratan, M.F. Haidere, Md. Nurunnabi, S.Md. Shahriar, Cancers 12(4) (2020) 855. https://www.mdpi.com/2072-6694/12/4/855
9 M.B. Baghirov, M. Muradov, G. Eyvazova, S. Mammadyarova, Y. Azizian-Kalandaragh, N. Musayeva, E.K. Gasimov, F.H. Rzayev, RSC Advances 14 (2024) 16696. https://doi.org/10.1039/D4RA01585A
10 K. Kumar, S.R. Anand, M. Kori, N. Mishra, S.P. Shrivastava, Journal of the Indian Chemical Society 100(4) (2023) 10096. https://doi.org/10.1016/j.jics.2023.100965
11 E.G. Karimli, V.N. Khrustalev, M.N. Kurasova, M. Akkurt, A.N. Khalilov, A. Bhattarai, I.G. Mamedov, Acta Crystallographica Section E 79(5) (2023) 474. https://doi.org/10.1107/S205698902300333X
12 F.N. Naghiyev, T.A. Tereshina, V.N. Khrustalev, M. Akkurt, A.N. Khalilov, A.A. Akobirshoeva, I.G. Mamedov, Acta Crystallographica Section E 77(5) (2021) 512. https://doi.org/10.1107/S2056989021003625
13 A.N. Khalilov, J. Cisterna, A. Cárdenas, B. Tuzun, S. Erkan, A.V. Gurbanov, I. Brito. Synthesis, Journal of Molecular Structure 1313 (2024) 138652. https://doi.org/10.1016/j.molstruc.2024.138652
14 V.G. Nenajdenko, A.A. Kazakova, A.S. Novikov, N.G. Shikhaliyev, A.M. Maharramov, A.M. Qajar, G.T. Atakishiyeva, A.A. Niyazova, V.N. Khrustalev, A.V. Shastin, A.G. Tskhovrebov, Catalysts 13(8) (2023) 1194. https://doi.org/10.3390%2Fcatal13081194
15 A. Maharramov, N.Q. Shikhaliyev, A. Abdullayeva, G.T. Atakishiyeva, A. Niyazova, V.N. Khrustalev, S.I. Gahramanova, Z. Atioğlu, M. Akkurt, A. Bhattarai, Acta Crystallographica Section E 79(10) (2023) 905. https://doi.org/10.1107/S2056989023007855
16 A. Maharramov, N.Q. Shikhaliyev, A. Qajar, G.T. Atakishiyeva, A. Niyazova, V.N. Khrustalev, M. Akkurt, S.Ö. Yıldırım, A. Bhattarai, Acta Crystallographica Section E 79(7) (2023) 637. https://doi.org/10.1107/S205698902300511X
17 T. Shi, Q. Wei, Zh. Wang, G. Zhang, X. Sun, Q.-Y. He, Molecular Biology and Physiology 4(3) (2019). https://doi.org/10.1128/msphere.00175-19
18 N. Aqila, N.H. Aprilita, D. Siswanta, Global NEST Journal 22(3) (2020) 408. https://doi.org/10.30955/gnj.003287
19 E.A. Badra, S.H. Shafeka, H.H.H. Hefnib, Journal of Molecular Liquids 326 (2021) 115342. https://doi.org/10.1016/j.molliq.2021.115342
20 H. Wang, G. Zhang, S. Mahmud, R. Mia, H. Liu, Journal of Alloys and Compounds 894 (2022) 162502. https://doi.org/10.1016/j.jallcom.2021.162502
21 E.A. Terenteva, V.V. Apyari, E. Kochuk, S. Dmitrienko, and Yu.A. Zolotov, J Anal Chem 72(11) (2017) 978. https://doi.org/10.1134/S1061934817110107
22 E.A. Terenteva, V.V. Apyari, S.G. Dmitrienko, Yu.A. Zolotov, Spectrochimica Acta Part 151 (2015) 89. http://dx.doi.org/10.1016/j.saa.2015.06.049
23 M. Pervaiza, S. Sadiqa, A. Sadiq, A. Ashraf, Z. Saeed, Coordination Chemistry Reviews 447 (2021) 214128. https://doi.org/10.1016/j.ccr.2021.214128
24 J. Swanner, C.D Fahrenholtz, I. Tenvooren, B.W. Bernish , J.J. Sears, A. Hooker, C.M. Furdui, E. Alli, W. Li, G.L. Donati, K.L. Cook, P.-A. Vidi, R. Singh, FASEB Bioadvances 1(10) (2019) 639. https://doi.org/10.1096/fba.2019-00021
25 M. Pervaiza, M. Shahina, A. Ejaza, R. Quratulaina, Z. Saeed, A. Ashraf, R.R.M. Khan, S.M. Bukhari, S. Ullah, U. Younas, Inorganic Chemistry Communications 159 (2024) 111851. https://doi.org/10.1016/j.inoche.2023.111851
A.A. İmamaliyeva, F.V. Hajiyeva, F.M. Ciraqov, Synthesis and structural characterization of new complexes based on silver nanoparticles, diphenylcarbazide, and cetyltrimethylammonium bromide, UNEC J. Eng. Appl. Sci. 4(2) (2024) 12-19. https://doi.org/10.61640/ujeas.2024.1202
Anyone you share the following link with will be able to read this content:

This article is licensed under the Creative Commons Attribution ( CC BY 4.0 ) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
W.M. Shume, H.C. Ananda Murthy, E.A. Zereffa, Journal of Chemistry 2020 (2020). https://doi.org/10.1155/2020/5039479
M. Gao, L. Sun, Zh. Wang, Y. Zhao, Materials Science and Engineering 33(1) (2013) 397. https://doi.org/10.1016/j.msec.2012.09.005
K.V. Alex, P.T. Pavai, R. Rugmini, M.S. Prasad, ACS Omega 5(22) (2020) 13123. https://dx.doi.org/10.1021/acsomega.0c01136
S. Shariati, Gh. Khayatian, RSC Advances (11) (2021) 3295. https://doi.org/10.1039/D0RA08615K
A. Sanchez-Hachair, A. Hofmann, Comptes Rendus Chimie 21(9) (2018) 890. https://doi.org/10.1016/j.crci.2018.05.002endus
V. Kumar, D. Singh, S. Mohan, D. Bano, R.K. Gundampati, Journal of Photochemistry and Photobiology B: Biology 168 (2017) 67. https://doi.org/10.1016/j.jphotobiol.2017.01.022
M.A. Farooqi, S. Bae, S. Kim, S. Bae, Scientific Reports 14(1) (2024) 22922. https://doi.org/10.1038/s41598-024-723565
Z.A. Ratan, M.F. Haidere, Md. Nurunnabi, S.Md. Shahriar, Cancers 12(4) (2020) 855. https://www.mdpi.com/2072-6694/12/4/855
M.B. Baghirov, M. Muradov, G. Eyvazova, S. Mammadyarova, Y. Azizian-Kalandaragh, N. Musayeva, E.K. Gasimov, F.H. Rzayev, RSC Advances 14 (2024) 16696. https://doi.org/10.1039/D4RA01585A
K. Kumar, S.R. Anand, M. Kori, N. Mishra, S.P. Shrivastava, Journal of the Indian Chemical Society 100(4) (2023) 10096. https://doi.org/10.1016/j.jics.2023.100965
E.G. Karimli, V.N. Khrustalev, M.N. Kurasova, M. Akkurt, A.N. Khalilov, A. Bhattarai, I.G. Mamedov, Acta Crystallographica Section E 79(5) (2023) 474. https://doi.org/10.1107/S205698902300333X
F.N. Naghiyev, T.A. Tereshina, V.N. Khrustalev, M. Akkurt, A.N. Khalilov, A.A. Akobirshoeva, I.G. Mamedov, Acta Crystallographica Section E 77(5) (2021) 512. https://doi.org/10.1107/S2056989021003625
A.N. Khalilov, J. Cisterna, A. Cárdenas, B. Tuzun, S. Erkan, A.V. Gurbanov, I. Brito. Synthesis, Journal of Molecular Structure 1313 (2024) 138652. https://doi.org/10.1016/j.molstruc.2024.138652
V.G. Nenajdenko, A.A. Kazakova, A.S. Novikov, N.G. Shikhaliyev, A.M. Maharramov, A.M. Qajar, G.T. Atakishiyeva, A.A. Niyazova, V.N. Khrustalev, A.V. Shastin, A.G. Tskhovrebov, Catalysts 13(8) (2023) 1194. https://doi.org/10.3390%2Fcatal13081194
A. Maharramov, N.Q. Shikhaliyev, A. Abdullayeva, G.T. Atakishiyeva, A. Niyazova, V.N. Khrustalev, S.I. Gahramanova, Z. Atioğlu, M. Akkurt, A. Bhattarai, Acta Crystallographica Section E 79(10) (2023) 905. https://doi.org/10.1107/S2056989023007855
A. Maharramov, N.Q. Shikhaliyev, A. Qajar, G.T. Atakishiyeva, A. Niyazova, V.N. Khrustalev, M. Akkurt, S.Ö. Yıldırım, A. Bhattarai, Acta Crystallographica Section E 79(7) (2023) 637. https://doi.org/10.1107/S205698902300511X
T. Shi, Q. Wei, Zh. Wang, G. Zhang, X. Sun, Q.-Y. He, Molecular Biology and Physiology 4(3) (2019). https://doi.org/10.1128/msphere.00175-19
N. Aqila, N.H. Aprilita, D. Siswanta, Global NEST Journal 22(3) (2020) 408. https://doi.org/10.30955/gnj.003287
E.A. Badra, S.H. Shafeka, H.H.H. Hefnib, Journal of Molecular Liquids 326 (2021) 115342. https://doi.org/10.1016/j.molliq.2021.115342
H. Wang, G. Zhang, S. Mahmud, R. Mia, H. Liu, Journal of Alloys and Compounds 894 (2022) 162502. https://doi.org/10.1016/j.jallcom.2021.162502
E.A. Terenteva, V.V. Apyari, E. Kochuk, S. Dmitrienko, and Yu.A. Zolotov, J Anal Chem 72(11) (2017) 978. https://doi.org/10.1134/S1061934817110107
E.A. Terenteva, V.V. Apyari, S.G. Dmitrienko, Yu.A. Zolotov, Spectrochimica Acta Part 151 (2015) 89. http://dx.doi.org/10.1016/j.saa.2015.06.049
M. Pervaiza, S. Sadiqa, A. Sadiq, A. Ashraf, Z. Saeed, Coordination Chemistry Reviews 447 (2021) 214128. https://doi.org/10.1016/j.ccr.2021.214128
J. Swanner, C.D Fahrenholtz, I. Tenvooren, B.W. Bernish , J.J. Sears, A. Hooker, C.M. Furdui, E. Alli, W. Li, G.L. Donati, K.L. Cook, P.-A. Vidi, R. Singh, FASEB Bioadvances 1(10) (2019) 639. https://doi.org/10.1096/fba.2019-00021
M. Pervaiza, M. Shahina, A. Ejaza, R. Quratulaina, Z. Saeed, A. Ashraf, R.R.M. Khan, S.M. Bukhari, S. Ullah, U. Younas, Inorganic Chemistry Communications 159 (2024) 111851. https://doi.org/10.1016/j.inoche.2023.111851