UNEC Journal of Engineering and Applied Sciences Volume 4, No 2, pages 47-54 (2024) Cite this article, 1519 https://doi.org/10.61640/ujeas.2024.1205
Recently, the study of nanoparticle added polymer structures are widely investigated by researcher due to new functional properties on nanosized materials such as flexibility, equal distribution etc. Like a another nanocrystalline or bulk crystals, the physical or chemical properties of these materials can be investigated by same methodologies [1-5]. In this regards, the study of thermal properties of polymers is very important from a scientific point of view. These properties provide an impetus both for polymer nanocomposites when exposed to high temperatures and for the selection of suitable material available. Natural and synthetic polymers are widely used in high-temperature fields of science, technology and industry. These materials, their processing includes pressing, extrusion, casting, etc. they are often exposed to the influence of temperature. In this regard, the qualitative and quantitative analysis of the physicochemical changes that occur during the effect of heat on the polymer is important [6-8]. Today, the scope of application of nanocomposites based on PP and nanoparticles is rapidly expanding. The inclusion of nanoparticles in the polymer matrix can have a positive effect on their thermostability. However, there are few similar properties between the inorganic nanoadditives and the polymer matrix in nanocomposites. Nanoparticles are characterized by a large surface area relative to the volume, so intermolecular interactions (electrostatic, magnetic, etc.) are stronger than micron-sized particles. This, in turn, can lead to the aggregation of nanoparticles, and as you can see, the dispersion of the nanofiller in the polymer matrix is quite complex. In this regard, the positive effect of metal nanoadditives on the thermal properties of the composite is somewhat limited. Thermal stability of a polymer mainly depends on its chemical structure, degree of crystallization and molecular weight. For example, it is known that aromatic structures in the polymer main chain increase its thermostability, while double bonds or oxygen-containing structures make it more resistant to high temperatures. In this study, PP was used as a polymer matrix, and MnO2 was used as a nanofiller. MnO2 nanofiller is an interesting material from several points of view. It is one of the few oxides that show quantum confinement effects in the experimentally accessible size range [19]. In addition, the wide range of morphological diversity in the nanoregime has made this material a promising candidate in the field of nanotechnologies and opened new opportunities for the production of high-performance devices based on these nanostructures [9-12]. Recently, several research papers on the preparation of PANI/MnO2 nanocomposites using the electrochemical method have been published [13-14]. In this work, we investigated the structural and thermal properties of PP/MnO2 nanocomposite.
2.1. Preparation of polymer nanocomposites
Obtaining a sample of the nanocomposite is carried out by hot pressing at a polymer melting temperature and a pressure of 15 MPa for 10 minutes. Nanocomposites obtained by the hot-pressing method are determined by the homogeneous distribution of the filler in the polymer matrix and the formation of defect-free structure. After hot pressing the films were cooled in water at room temperature. Film thicknesses is 120 μm.
2.2. X-ray diffractometry method
X-ray phase studies of nanocomposites are performed on a D2 Phaser X-ray diffractometer in reflection mode (Bragg-Brentano geometry) using CuKa radiation (average wavelength λ = 1.5406 A°, nickel β-filter). Registration is carried out in a continuous mode in the range of angles 2θ = 5–80°.
2.3. AFM scanning probe microscope
The description of topography and roughness of nanocomposite surface relief has been studied by AFM scanning probe microscope Integra-Prima (NT-MDT, Zelenograd). Scanning is carried out under air conditions in semi-contact mode with probes made by plasma chemical method with resonance frequency 1-5Hz and radius of tip curvature equal to 20 nm. Scanning speed and number of scanned lines in the image are 1.969 Hz and 256, respectively.
2.4. Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry (DSC) analyzes experiments were performed in the temperature range T=20÷300°C with a STA3000 Synchronous Thermal Analyzer device at a heating rate of 20°C/min. (figure1). Experimentally obtained DSC curves represent the dependence of heat flux (mJ/s) or specific heat (J/gK) on temperature. Heating in nitrogen atmosphere at the predetermined rate according to the controlled program is taken place and during the measurement process the flux of heat of the reference and the material under consideration is compared.
X-ray analyses serve as a cornerstone in various scientific fields, enabling the determination of crystal structures, material properties, and chemical compositions [19-25].Given X-ray analyses are used to determine crystallinity and to study the effect of MnO2 nanoparticle size and content on PP/MnO2 nanocomposite crystal structure (Figure 2). X-ray diffraction analysis shows that MnO2 nanoparticle introduction into PP matrix brings about the change in polymer matrix structure. Spectra of PP/0,5÷1 % MnO2 nanocomposites (figure 2a) are different from each other only by reducing peak intensity for nanocomposite samples. PP/MnO2 has a crystal phase according to the file JCPDF (MnO2 No.44-0141), where one can see diffraction peaks at 2θ: 27.65°(120), 29.82°(031), 37.78°(300), 44.21°(002), 50.32°(160), 61.37°(421). Peaks are in good agreement with MnO2 tetragonal phase structure. However diffraction peaks also show up at 2θ:13.8° (110), 16.6° (040), 15.8° (300) and 18.3°(130) that correspond in PP semicrystal structure. The position of diffraction lines for nanocomposites changes depending on the con centration. Strong peaks are predominantly observed for PP/1%MnO2 nanocomposite on the diffractogram.
Average size of PP/MnO2 prepared sample crystallites has been calculated by using Debye-Scherer formula:
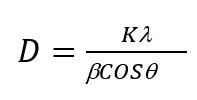 (1)
(1)
where D- is the particle size, λ = 1,5406˚A, β is the width of peak on FWHM, θ- is the diffraction angle (in radians), K- is the constant about 0.9. It is established that the average size of crystallites for PP/0,5% MnO2 is 28 nm, PP/1%MnO2 is 34.4 nm. Fall in phase transition is considered to be due to the presence of interface multitude resulting in particle size decrease.
It is known that the surface of polymer materials consists of irregularly located recesses and protrusions[26-30]. The degree of surface relief or the degree of roughness is a quantity that characterizes how many times the actual surface of materials differs from the ideal surface. The more transparent and smooth the surface of polymer samples, the better its mechanical-deformation properties. AFM is one of the main methods for determining the degree of surface roughness of polymer materials.
The description of the topography and roughness of the surface relief of PP/MnO2 nanocomposites was studied by means of a scanning probe AFM microscope. Figures 3 and 4 show 2D, 3D AFM images and histograms of relief of PP/0.5% MnO2and PP/1% MnO2 nanocomposites. It was determined that when MnO2 nanoparticles are present in polypropylene in the amount of 0.5%, in contrast to the amount of nanoparticles in the amount of 1%, a more regular supramolecular structure is formed. As can be seen from the AFM images, agglomeration of nanoparticles occurs in the matrix with 1% of the amount of manganese nanoparticles.
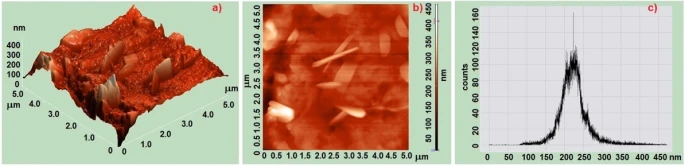
Figure 3. AFM images and histograms of PP-based and PP/0,5%MnO2 polymer nanocomposites: a) 3D; b) 2D; c) histograms PP/0,5%MnO2
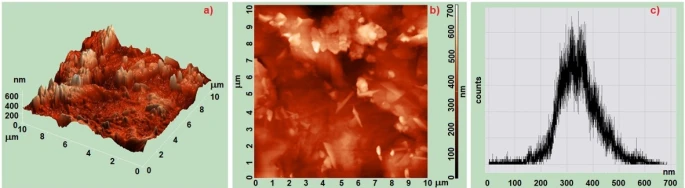
Figure 4. AFM images and histograms of PP-based and PP/1%MnO2 polymer nanocomposites: a) 3D; b) 2D; c) histograms PP/1%MnO2
Figure 3c and figure 4c show the histograms of the root mean square roughness obtained from AFM analysis of the surface of PP/ MnO2 based nanocomposites. It was determined that the mean square roughness of the PP/0.5% MnO2-based nanocomposite surface is 150-300 nm; For PP+1%MnO2-based nanocomposite, it is 200-500 nm. From this, it was concluded that a more regular structure is formed in small amounts of MnO2 nanoparticles in the polymer matrix, in contrast to high amounts of nanoparticles. As shown in figure 3, the surface of the PP/1%MnO2 nanocomposite is not smooth and has many defects. Thus, the centers of crystallization in polypropylene lost. Therefore, with the increase in the volume of MnO2 in the matrix, the probability of the agglomeration process of the nanocomposite increases.
Therefore, the uniform distribution of nanoparticles throughout the volume of nanocomposites and the effective surface area decrease. However, when polymers are modified into a nanocomposite, an orderly arrangement is obtained in small percentages of the nanocomposite.
Differential scanning calorimetry (DSC) is a thermal technique in which the difference in heat flux into a substance and a reference is measured as a function of sample temperature while both are subjected to a controlled temperature program [15-20]. Figure 5 shows the DSC curves, which were recorded in the temperature range from 20 oC to 250 °C at a heating rate of 20 °C/min. The melting temperature of the nanocomposites were recorded using the DSC thermograms. The melting temperature corresponding to the top of the peak on the DSC curve is taken as the melting temperature. The obtained thermal parameters are summarized in table 1. The weight of all samples was taken to be about 35-38 mg. As can be seen, the obtained curves of all samples are accompanied by an endothermic peak.
In addition, the degree of crystallization of PP/ MnO2 based polymer nanocomposites was calculated according to expression (2) (the value of ∆H0 for PP polymer was taken as 59C/g). The degree of crystallization of PP/MnO2 nanocomposites was calculated using the formula (2) using the melting enthalpy, which depends on the share of the crystalline part in the structure:
EQUATION
Here, ∆H is the melting enthalpy of the sample. ∆Hlit is the table value and is the melting enthalpy of a polymer with 100% crystalline structure.
The DSC thermograms show that the thermal stability of PP/0.5-1%MnO2 nanocomposites increases with the addition of MnO2 content. Polypropylene (PP) has a melting peak at 167.16 °C. In the case of 1% MnO2nanoadditive, the first endothermic maximum at 144.41 °C and the second additional melting peak at a temperature of about 209.41 °C appear in the PP/1%MnO2 nanocomposite, which corresponds to the melting of the dispersed PP phase. The endothermic maximum in the PP/0.5%MnO2 nanocomposite was found at 141.15 °C. The melting peak of the PP/1%MnO2 nanocomposite decreases by 22.75 °C compared to pure PP, and PP/0.5%MnO2, by 26.01°C. The second peak of the PP/1%MnO2 nanocomposite increases the melting temperature by 42.25°C compared to pure PP.
The degree of crystallinity determined by the DSC method takes into account the thermal processes occurring during the transition of the crystalline phase to the amorphous one. In this case, the enthalpy of melting characterizes the amount of energy spent on this transition. There is a difference in the values of the enthalpy of melting and the degree of crystallinity of nanocomposites compared to the original PP. This dependence reflects the combined effect on the degree of crystallinity of the matrix. With an increase in the content of the MnO2 nanofiller, the degree of crystallinity decreases, which indicates a decrease in the mobility of the polymer macromolecules in the melt. It is known that in highly filled systems, the adsorption interaction of the high-energy surface of the solid and macromolecules in the polymer melt leads to a limitation of their thermal mobility.
It is established that with the introduction of 1% amounts of MnO2 the physical properties of PP/MnO2 nanocomposite increase and the change in polymer matrix structure is taken place. PP/MnO2 nanocomposite polymer thermal properties are distinctly affected by the supramolecular structure of polymer (spherulite size, crystallinity degreeetc.) and interphase interaction at the interface. MnO2 metal-containing nanoparticles located at the boundary of interphase layer of PP structural elements make for forming heterogeneous nucleation centers in nanocomposition melting. The thermophysical parameters of nanocomposite materials change depending on the concentration. In addition, the enthalpy of melting in PP/1%MnO2 of the first and second endothermic maxima decreases. Also, in our opinion, this is due to the formation of changes in the supramolecular structure at the matrix-filler interface, and this factor is decisive in the transfer of thermal energy to the filler particles through the polymer phase.
The importance of this paper is that besides using PP/MnO2based nanocomposites as microelectronics for making memory cells, it is also possible to make new sensors based on these sample.
1 M.S. Leanenia, E.V. Lutsenko, M.V. Rzheutski, G.P. Yablonskii, T.G. Naghiyev, O.B. Tagiev, Journal of Applied Physics 129 (2021) 243104. https://doi.org/10.1063/5.0051319
2 R.S. Madatov, A.S. Alekperov, N.N. Gadzhieva, F.G. Asadov, Sh.A. Allahverdiev, E.G. Asadov, T.G. Naghiyev, International Journal of Modern Physics B 33(09) (2019) 1950066. https://doi.org/10.1142/S0217979219500668
3 R.F. Babayeva, T.G. Naghiyev, Modern Physics Letters B 37(21) (2023) 2350058. https://doi.org/10.1142/S0217984923500586
4 A.P. Abdullayev, R.M. Rzayev, T.G. Naghiyev, J.P. Mammadova, S.S. Aliyev, I.V. Musazade, International Journal of Modern Physics B 37(28) (2023) 2350248. https://doi.org/10.1142/S021797922350248X
5 T.G. Naghiyev, R.F. Babayeva, Y.I. Aliyev. The European Physical Journal B 97(6) (2024) 86. https://doi.org/10.1140/epjb/s10051-024-00731-2
6 H. Donya, T.A. Taha, A. Alruwaili, I.B.I. Tomsah, M. Ibrahim, Journal of Materials Research and Technology 9(4) (2020) 9189. https://doi.org/10.1016/j.jmrt.2020.06.040
7 T.A.M. Taha, K. AlanaziSSSEl-Nasser, A.H. Alshammari, A. Ismael, Polymers 16(6) (2024) 736.
8 T.A. Taha, M.H. Mahmoud, H.H. Hamdeh, Journal of Polymer Research 28(5) (2021) 148. http://dx.doi.org/10.1007/s10965-021-02508-y
9 U. Koch, A. Fojtik, H. Weller, Chemical Physics Letters 122(5) (1985) 507. https://doi.org/10.1016/0009-2614(85)87255-9
10 C. Klingshrin, ChemPhysChem 8(6) (2007) 782. https://doi.org/10.1002/cphc.200700002
11 Z.L. Wang, Materials Today 7(6) (2004) 26. https://doi.org/10.1016/S1369-7021(04)00286-X
12 Y. Cui, Q.Q. Wei, H.K. Park, C.M. Libera, Science 293(5533) (2001) 1289.
13 T.A. Taha, M.A.A. Alzara, Journal of Molecular Structure 1238 (2021) 130401. https://doi.org/10.1016/j.molstruc.2021.130401
14 S. Alhassan, K. Alshammari, M. Alshammari, T. Alotaibi, A.H. Alshammari, A. Alhamazani, T.A.M. Taha, Results in Physics 58 (2024) 107456. https://doi.org/10.1016/j.rinp.2024.107456
15 A.H. Alshammari, S. Alhassan, A. Iraqi, S.A. Saad, T.A.M. Taha, Journal of Materials Science: Materials in Electronics 34 (2023) 2132. https://doi.org/10.1007/s10854-023-11548-7
16 W.S. Kim, H.S. Song, B.O. Lee, K.H. Kwon, Y.S. Lim, M.S. Kim, Macromolecular Research 10 (2002) 253. https://doi.org/10.1007/BF03218314
17 P. Barber, S. Balasubramanian, Y.Anguchamy, S. Gong, A. Wibowo, H. Gao, H.J. Ploehn, H.C. Zur Loye, Materials 2(4) (2009) 1697. https://doi.org/10.3390/ma2041697
18 A.S. Huseynova, R.M. Rzayev, F.V. Hajiyeva, Journal of the Korean Physical Society 85 (2024) 76. https://doi.org/10.1007/s40042-024-01094-8
19 E.G. Karimli, V.N. Khrustalev, M.N. Kurasova, M. Akkurt, A.N. Khalilov, A. Bhattarai, I.G. Mamedov, Acta Crystallographica Section E 79(5) (2023) 474. https://doi.org/10.1107/S205698902300333X
20 F.N. Naghiyev, T.A. Tereshina, V.N. Khrustalev, M. Akkurt, A.N. Khalilov, A.A. Akobirshoeva, I.G. Mamedov, Acta Crystallographica Section E 77(5) (2021) 512. https://doi.org/10.1107/S2056989021003625
21 A.N. Khalilov, J. Cisterna, A. Cárdenas, B. Tuzun, S. Erkan, A. V. Gurbanov, I. Brito. Synthesis, Journal of Molecular Structure 1313 (2024) 138652. https://doi.org/10.1016/j.molstruc.2024.138652
22 V.G. Nenajdenko, A.A. Kazakova, A.S. Novikov, N.G. Shikhaliyev, A.M. Maharramov, A.M. Qajar, G.T. Atakishiyeva, A.A. Niyazova, V.N. Khrustalev, A.V. Shastin, A.G. Tskhovrebov, Catalysts 13(8) (2023) 1194. https://doi.org/10.3390/catal13081194
23 A. Maharramov, N.Q. Shikhaliyev, A. Abdullayeva, G.T. Atakishiyeva, A. Niyazova, V.N. Khrustalev, S.I. Gahramanova, Z. Atioğlu, M. Akkurt, A. Bhattarai, Acta Crystallographica Section E 79(10) (2023) 905. https://doi.org/10.1107/S2056989023007855
24 Z. Atioglu, M. Akkurt, N.Q. Shikhaliyev, U.F. Askerova, A.A. Niyazova, S. Mlowe, Acta Crystallographica Section E 77(8) (2021) 829. https://doi.org/10.1107/S2056989021007349
25 R.K. Askerov, A.M. Maharramov, A.N. Khalilov, M. Akkurt, A.A. Akobirshoeva, V.K. Osmanov, A.V. Borisov, Acta Crystallographica Section E 76(7) (2020) 1007. https://doi.org/10.1107/S2056989020007033
26 M.L. Saladino, T.E Motaung, A.S. Luyt, A. Spinella, G. Nasillo, E. Caponetti, Polymer Degradation and Stability 97(3) (2012) 452. https://doi.org/10.1016/j.polymdegradstab.2011.11.006
27 A.S. Huseynova, R.M. Rzayev, F.V. Hajiyeva, Journal of Elastomers & Plastics 56(8) (2024) 929. https://doi.org/10.1177/00952443241289560
28 K. Król-Morkisz, K. Pielichowska, Polymer Composites with Functionalized Nanoparticles (2019) 405. https://doi.org/10.1016/B978-0-12-814064-2.00013-5
29 M.A. Ramazanov, A. Huseynova, N. Eyubova, S.A. Abasov, Journal Optoelectronics and Advanced Materials 4(12) (2010) 2003.
30 S. Dul, L. Fambri, A. Pegoretti, Nanomaterials 8(1) (2018) 4. https://doi.org/10.3390/nano8010049
A.S. Huseynova, R.R. Bekmirzayev, G.A. Muradova, Morphology and thermal properties of a nanocomposite based on polypropylene PP/MnO2, UNEC J. Eng. Appl. Sci. 4(2) (2024) 47-54 https://doi.org/10.61640/ujeas.2024.1205
Anyone you share the following link with will be able to read this content:

This article is licensed under the Creative Commons Attribution ( CC BY 4.0 ) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
M.S. Leanenia, E.V. Lutsenko, M.V. Rzheutski, G.P. Yablonskii, T.G. Naghiyev, O.B. Tagiev, Journal of Applied Physics 129 (2021) 243104. https://doi.org/10.1063/5.0051319
R.S. Madatov, A.S. Alekperov, N.N. Gadzhieva, F.G. Asadov, Sh.A. Allahverdiev, E.G. Asadov, T.G. Naghiyev, International Journal of Modern Physics B 33(09) (2019) 1950066. https://doi.org/10.1142/S0217979219500668
R.F. Babayeva, T.G. Naghiyev, Modern Physics Letters B 37(21) (2023) 2350058. https://doi.org/10.1142/S0217984923500586
A.P. Abdullayev, R.M. Rzayev, T.G. Naghiyev, J.P. Mammadova, S.S. Aliyev, I.V. Musazade, International Journal of Modern Physics B 37(28) (2023) 2350248. https://doi.org/10.1142/S021797922350248X
T.G. Naghiyev, R.F. Babayeva, Y.I. Aliyev. The European Physical Journal B 97(6) (2024) 86. https://doi.org/10.1140/epjb/s10051-024-00731-2
H. Donya, T.A. Taha, A. Alruwaili, I.B.I. Tomsah, M. Ibrahim, Journal of Materials Research and Technology 9(4) (2020) 9189. https://doi.org/10.1016/j.jmrt.2020.06.040
T.A.M. Taha, K. AlanaziSSSEl-Nasser, A.H. Alshammari, A. Ismael, Polymers 16(6) (2024) 736.
T.A. Taha, M.H. Mahmoud, H.H. Hamdeh, Journal of Polymer Research 28(5) (2021) 148. http://dx.doi.org/10.1007/s10965-021-02508-y
U. Koch, A. Fojtik, H. Weller, Chemical Physics Letters 122(5) (1985) 507. https://doi.org/10.1016/0009-2614(85)87255-9
C. Klingshrin, ChemPhysChem 8(6) (2007) 782. https://doi.org/10.1002/cphc.200700002
Z.L. Wang, Materials Today 7(6) (2004) 26. https://doi.org/10.1016/S1369-7021(04)00286-X
Y. Cui, Q.Q. Wei, H.K. Park, C.M. Libera, Science 293(5533) (2001) 1289.
T.A. Taha, M.A.A. Alzara, Journal of Molecular Structure 1238 (2021) 130401. https://doi.org/10.1016/j.molstruc.2021.130401
S. Alhassan, K. Alshammari, M. Alshammari, T. Alotaibi, A.H. Alshammari, A. Alhamazani, T.A.M. Taha, Results in Physics 58 (2024) 107456. https://doi.org/10.1016/j.rinp.2024.107456
A.H. Alshammari, S. Alhassan, A. Iraqi, S.A. Saad, T.A.M. Taha, Journal of Materials Science: Materials in Electronics 34 (2023) 2132. https://doi.org/10.1007/s10854-023-11548-7
W.S. Kim, H.S. Song, B.O. Lee, K.H. Kwon, Y.S. Lim, M.S. Kim, Macromolecular Research 10 (2002) 253. https://doi.org/10.1007/BF03218314
P. Barber, S. Balasubramanian, Y.Anguchamy, S. Gong, A. Wibowo, H. Gao, H.J. Ploehn, H.C. Zur Loye, Materials 2(4) (2009) 1697. https://doi.org/10.3390/ma2041697
A.S. Huseynova, R.M. Rzayev, F.V. Hajiyeva, Journal of the Korean Physical Society 85 (2024) 76. https://doi.org/10.1007/s40042-024-01094-8
E.G. Karimli, V.N. Khrustalev, M.N. Kurasova, M. Akkurt, A.N. Khalilov, A. Bhattarai, I.G. Mamedov, Acta Crystallographica Section E 79(5) (2023) 474. https://doi.org/10.1107/S205698902300333X
F.N. Naghiyev, T.A. Tereshina, V.N. Khrustalev, M. Akkurt, A.N. Khalilov, A.A. Akobirshoeva, I.G. Mamedov, Acta Crystallographica Section E 77(5) (2021) 512. https://doi.org/10.1107/S2056989021003625
A.N. Khalilov, J. Cisterna, A. Cárdenas, B. Tuzun, S. Erkan, A. V. Gurbanov, I. Brito. Synthesis, Journal of Molecular Structure 1313 (2024) 138652. https://doi.org/10.1016/j.molstruc.2024.138652
V.G. Nenajdenko, A.A. Kazakova, A.S. Novikov, N.G. Shikhaliyev, A.M. Maharramov, A.M. Qajar, G.T. Atakishiyeva, A.A. Niyazova, V.N. Khrustalev, A.V. Shastin, A.G. Tskhovrebov, Catalysts 13(8) (2023) 1194. https://doi.org/10.3390/catal13081194
A. Maharramov, N.Q. Shikhaliyev, A. Abdullayeva, G.T. Atakishiyeva, A. Niyazova, V.N. Khrustalev, S.I. Gahramanova, Z. Atioğlu, M. Akkurt, A. Bhattarai, Acta Crystallographica Section E 79(10) (2023) 905. https://doi.org/10.1107/S2056989023007855
Z. Atioglu, M. Akkurt, N.Q. Shikhaliyev, U.F. Askerova, A.A. Niyazova, S. Mlowe, Acta Crystallographica Section E 77(8) (2021) 829. https://doi.org/10.1107/S2056989021007349
R.K. Askerov, A.M. Maharramov, A.N. Khalilov, M. Akkurt, A.A. Akobirshoeva, V.K. Osmanov, A.V. Borisov, Acta Crystallographica Section E 76(7) (2020) 1007. https://doi.org/10.1107/S2056989020007033
M.L. Saladino, T.E Motaung, A.S. Luyt, A. Spinella, G. Nasillo, E. Caponetti, Polymer Degradation and Stability 97(3) (2012) 452. https://doi.org/10.1016/j.polymdegradstab.2011.11.006
A.S. Huseynova, R.M. Rzayev, F.V. Hajiyeva, Journal of Elastomers & Plastics 56(8) (2024) 929. https://doi.org/10.1177/00952443241289560
K. Król-Morkisz, K. Pielichowska, Polymer Composites with Functionalized Nanoparticles (2019) 405. https://doi.org/10.1016/B978-0-12-814064-2.00013-5
M.A. Ramazanov, A. Huseynova, N. Eyubova, S.A. Abasov, Journal Optoelectronics and Advanced Materials 4(12) (2010) 2003.
S. Dul, L. Fambri, A. Pegoretti, Nanomaterials 8(1) (2018) 4. https://doi.org/10.3390/nano8010049