UNEC Journal of Engineering and Applied Sciences Volume 3, No 1, pages 28-32 (2023) Cite this article, 1347 https://doi.org/10.61640/ujeas.2023.0505
Chalcogenide semiconductors are widely used in electronics for various purposes. Depending on the energy band gap, it is possible to make different converters from them. Therefore, various studies are being conducted to study the physical and chemical properties of these materials. It was determined that the structural features and defects of chalcogenide semiconductors affect the formation of their physical properties. Therefore, the structural properties of these materials (crystal and electronic structure) are widely studied [1-5].
The GeS compound occupies a special place among chalcogenide semiconductors. It was determined that interesting optical properties can be observed when replacements are made with rare earth elements in this compound [6, {ref;7}]. It is known that during substitutions, certain changes occur in the crystal structure depending on the ionic radii of metal atoms. These changes also affect the electronic structures of crystals. Therefore, changes in many physical properties of samples occur during partial substitutions. Especially it has a strong effect on magnetic and electrical properties. That is why, partial substitutions are made for obtaining new functional materials on the basis of known compounds. However, during these substitutions, the ionic radii and valences of atoms should be taken into account 8-10].
Physical properties of semiconductors are depends on the temperature. As the temperature increases, electrical conductivity increases due to the increase of additional charge carriers [11]. Therefore, it is important to study the thermal properties of the materials. It was determined that the method of Differential Thermal Analysis allows to study the thermal processes occurring in semiconductors with high accuracy. Through these methods, it is possible to determine thermodynamic parameters: Wigner potential, Gibbs potential, free energy, enthalpy [12]. Although many physical properties of solid solutions of GeS compound have been studied, thermal processes in Ge0.99Nd0.01S compound have not been sufficiently studied. In this work, the thermal properties of the Ge0.99Nd0.01S compound were studied in detail.
Germanium with a resistance of 50 Ohm·cm, sulfur (B5) and neodymium (Nd-2) were taken as starting materials. A stoichiometric mixture of these elements is placed in a quartz tube. A 10-3 mm Hg vacuum was obtained in a quartz tube. To prevent the explosion of the ampoule, the germanium was powdered and the amount of the substance was limited to about 10-15 g. The synthesis process was carried out in two stages. In the first stage, the ampoule was kept in the furnace and heated. The temperature was raised to 300 0C at a rate of 3-5 0C/min and maintained for 10-12 hours. At the next stage, the furnace temperature was raised to 1000 0C at a rate of 2-3 0C/min and this temperature was maintained for 18-20 hours. After that, the furnace was cooled down to room temperature with the sample. The structure of the polycrystal of the synthesized Ge0.99Nd0.01S compound was studied on a D8 ADVANCE X-ray diffractometer and it was found that a single-phase sample was obtained.
The thermophysical properties of the Ge0.99Nd0.01S compound with a purity of 99.99% and a density of 2.56 g/cm3 were studied by the DSC method in the temperature range of T = 25-800 0C. DSC measurements were performed on a TGA/DSC3+ instrument manufactured by METTLER TOLEDO and temperature control was performed using MULTISTAR sensors. A standard adiabatic calorimeter was performed in the temperature range from 25 0C to 800 0C in an argon (Ar) atmosphere at a heating rate of 20 ml/min, 5 0/min. The cooling process was achieved with the NITROGEN UN 1977 SOFRIGERATED LIQUID analyzer cooling system and "digital temperature controller".
Figure 1 shows the differential heat flux and heat flux spectra of the alloy in the Ge0.99Nd0.01S compound temperature range 25 0C ≤ T ≤ 800 0C. Data measured at 3654 points for heat flux are given in the spectra. Despite the separation of the heat flux into two parts depending on the temperature, the differentiated spectrum of the heat flux clearly includes structural transformations, phase transitions and decomposition processes.
Figure 1. Heat flux and differential heat flux spectrum of Ge0.99Nd0.01S compound in the temperature range 25 0C ≤ T ≤ 750 0C
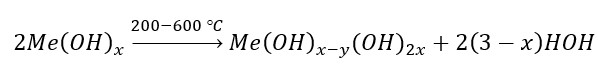
Figure 2 shows the areas of effects characterizing the structural transformations, phase transitions and decomposition processes occurring in the Ge0.99Nd0.01S compound in the temperature interval 25 0C ≤ T ≤ 800 0C.
Figure 2. Areas of transitions occurring in the Ge0.99Nd0.01S compound in the temperature range 25 0C ≤ T ≤ 800 0C
The Ge0.99Nd0.01S compound was synthesized with partial replacements of Ge atoms with Nd atoms in the GeS compound. The thermophysical properties of polycrystals of this compound were studied at high temperatures. It was determined that this compound contains water in a suspended state in the form of free and hydroxide groups. As the temperature rises, the hydroxide groups decomposited and the water molecules leave the sample. For these thermal effects, the temperature value and the area of the effect were evaluated. At higher temperatures, melting occurred in the sample and transition to liquid state occurred at 596 0C. As a result of thermogravimetric analysis, it was determined that during melting, the mass of the sample significantly decreases. This process is explained by the fact that high-energy atoms on the surface leave the sample.
1 R.F. Hashimov, N.A. Ismayilova, F.A. Mikailzade, A.O. Dashdemirov, A.V. Trukhanov, S.V. Trukhanov, Y.I. Aliyev, E.B. Asgerov, S.H. Jabarov, N.T. Dang, Modern Physics Letters B 32 (2018) 1850186.
2 Y.I. Aliyev, Y.G. Asadov, R.D. Aliyeva, T.G. Naghiyev, S.H. Jabarov, Modern Physics Letters B 33(11) (2019) 1850128.
3 E.M. Huseynov, T.G. Naghiyev, U.S. Aliyeva, Physica B: Condensed Matter 577 (2020) 411788 .
4 E.M. Huseynov, T.G. Naghiyev, Vaccum 212 (2023) 111990.
5 T.G. Naghiyev, Modern Physics Letters B 37(12) (2023) 2350021.
6 T.G. Naghiyev, U.R. Rzayev, E.M. Huseynov, I.T. Huseynov, S.H. Jabarov, UNEC J. Eng. Appl. Sci 2(1) (2022) 85.
7 A.O. Dashdemirov, A.S. Alekperov, N.A. Ismayilova, A.A. Hadieva, A.N. Jafarova, A.S. Abiyev, Advanced Physical Research 5 (2023) 12.
8 G.Sh. Ayyubova, S.H. Jabarov, UNEC J. Eng. Appl. Sci. 1(1) (2021) 49.
9 9. M.N. Mirzayev, Kh.F. Mammadov, V.A. Skuratov, E. Demir, S.H. Jabarov, N.A. Ismayilova, S. Biira, B. Abdurakhimov, E. Popov, Journal of Alloys and Compounds 801 (2019) 151.
10 F.G. Agayev, S.H. Jabarov, G.Sh. Ayyubova, M.N. Mirzayev, S.V. Trukhanov, E.L. Trukhanova, M.A. Darwish, S.V. Podgornaya , D.A. Vinnik, T.P. Hoang, N.T. Dang, A.V. Trukhanov, Physica B: Condensed Matter 580 (2020) 411772.
11 S.H. Jabarov, V.B. Aliyeva, T.G. Mammadov, A.I. Mammadov,S.E. Kichanov, L.S. Dubrovinsky, S.S. Babayev, E.G. Pashayeva, N.T. Dang, Materials Science-Poland 36(2) (2018) 203.
12 T.G. Naghiyev, R.M. Rzayev, Modern Physics Letters B 35(31) (2021) 2150469.
13 S.H. Jabarov, S.I. Ibrahimova, F.V. Hajiyeva, E.M. Huseynov, Y.I. Aliyev, Arabian Journal for Science and Engineering 47 (2022) 7817.
14 Y.I. Aliyev, Y.G. Asadov, T.M. Ilyasli, F.M. Mammadov, T.G. Naghiyev, Z.A. Ismayilova, M.N. Mirzayev, S.H. Jabarov, Modern Physics Letters B 34 (2020) 2050066.
A.O. Dashdemirov, A.S. Alekperov, Y.I. Aliyev, Mechanism and kinetics of thermal processes in Ge0.99Nd0.01S compound, UNEC J. Eng. Appl. Sci. 3(1) (2023) 28-32 https://doi.org/10.61640/ujeas.2023.0505
Anyone you share the following link with will be able to read this content:

This article is licensed under the Creative Commons Attribution ( CC BY 4.0 ) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
R.F. Hashimov, N.A. Ismayilova, F.A. Mikailzade, A.O. Dashdemirov, A.V. Trukhanov, S.V. Trukhanov, Y.I. Aliyev, E.B. Asgerov, S.H. Jabarov, N.T. Dang, Modern Physics Letters B 32 (2018) 1850186.
Y.I. Aliyev, Y.G. Asadov, R.D. Aliyeva, T.G. Naghiyev, S.H. Jabarov, Modern Physics Letters B 33(11) (2019) 1850128.
E.M. Huseynov, T.G. Naghiyev, U.S. Aliyeva, Physica B: Condensed Matter 577 (2020) 411788 .
E.M. Huseynov, T.G. Naghiyev, Vaccum 212 (2023) 111990.
T.G. Naghiyev, Modern Physics Letters B 37(12) (2023) 2350021.
T.G. Naghiyev, U.R. Rzayev, E.M. Huseynov, I.T. Huseynov, S.H. Jabarov, UNEC J. Eng. Appl. Sci 2(1) (2022) 85.
A.O. Dashdemirov, A.S. Alekperov, N.A. Ismayilova, A.A. Hadieva, A.N. Jafarova, A.S. Abiyev, Advanced Physical Research 5 (2023) 12.
G.Sh. Ayyubova, S.H. Jabarov, UNEC J. Eng. Appl. Sci. 1(1) (2021) 49.
9. M.N. Mirzayev, Kh.F. Mammadov, V.A. Skuratov, E. Demir, S.H. Jabarov, N.A. Ismayilova, S. Biira, B. Abdurakhimov, E. Popov, Journal of Alloys and Compounds 801 (2019) 151.
F.G. Agayev, S.H. Jabarov, G.Sh. Ayyubova, M.N. Mirzayev, S.V. Trukhanov, E.L. Trukhanova, M.A. Darwish, S.V. Podgornaya , D.A. Vinnik, T.P. Hoang, N.T. Dang, A.V. Trukhanov, Physica B: Condensed Matter 580 (2020) 411772.
S.H. Jabarov, V.B. Aliyeva, T.G. Mammadov, A.I. Mammadov,S.E. Kichanov, L.S. Dubrovinsky, S.S. Babayev, E.G. Pashayeva, N.T. Dang, Materials Science-Poland 36(2) (2018) 203.
T.G. Naghiyev, R.M. Rzayev, Modern Physics Letters B 35(31) (2021) 2150469.
S.H. Jabarov, S.I. Ibrahimova, F.V. Hajiyeva, E.M. Huseynov, Y.I. Aliyev, Arabian Journal for Science and Engineering 47 (2022) 7817.
Y.I. Aliyev, Y.G. Asadov, T.M. Ilyasli, F.M. Mammadov, T.G. Naghiyev, Z.A. Ismayilova, M.N. Mirzayev, S.H. Jabarov, Modern Physics Letters B 34 (2020) 2050066.