UNEC Journal of Engineering and Applied Sciences Volume 2 No 1, pages 33-40 (2022) Cite this article, 2740
Free radical molecules have unpaired electrons and produced during different oxidation reactions, which are highly unstable and reactive, damaging to nucleic acids, lipids and proteins via chain reactions. Therefore, results in pathogenesis of various disorders like diabetes, cardiovascular and autoimmune diseases, neurodegenerative disorders and aging processes. Antioxidants interact with free radicals and terminate the chain reactions. They act like free radical scavenger agents and play an important role to prevent the damage of free radicals [1,2]. Various foods contain compounds that act like antioxidants. In this study, the antioxidant activities of seven compounds (Figure 1) such as quercetin, ascorbyl palmitate, p-coumaric acid, butylated hydroxyanisole, dodecyl gallate, ferulic acid and kaempferol that act as free radical scavengers are investigated and ranked.
Figure 1. The chemical structures and the numbering of their hydroxyl groups: (1) R=OH quercetin and R=H kaempferol, (2) ferulic acid, (3) p-coumaric acid, (4) ascorbyl palmitate, (5) dodecyl gallate and (6) 2-BHA and 3-BHA.
Quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one) is the most popular flavonoid, it has its widest existence in fruits and vegetables especially onions, broccoli and red wine. Ascorbyl palmitate is a fat-soluble form of ascorbic acid (vitamin C). It can be absorbed also stored in the body and converted to ascorbic acid by the enzyme esterase. p-Coumaric acid (trans-4-hydroxycinnamic acid), a phenolic acid, is a hydroxyl derivative of cinnamic acid and ubiquitously contained in vegetables (e.g. potatoes) and fruits (e.g. apples, grapes, pears)2. Butylated hydroxyanisole (BHA) is a phenolic antioxidant that is widely used as a synthetic food additive to preserve oils and fats [3]. Dodecyl gallate (Lauryl gallate) is widely used as a scavenger of reactive oxygen [4,5]. Ferulic acid (4-hydroxy-3-methoxycinnamic acid) is found in seeds, leaves and conjugated to the plant cell wall polysaccharides, glycoproteins [6,7]. Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) is a flavonol that exists in many herbs and fruits such as tea, strawberries and grapes [8,9]. These compounds present several biological effects like antiviral, anti-inflammatory, antimicrobial, antiallergic, and anticancer. They also act as a superoxide scavenger therefore exhibits antioxidant activity.
In this study, quantum chemical approaches have been used to investigate the eight phenolic compounds’ antioxidant activities. The anti-oxidation mechanisms and molecular descriptors of the compounds were analyzed by theoretical methods with density functional theory (DFT) M06-2X method 6-311+G(d,p) and 6-31+G(d,p) basis sets in gas and water phases.
All compounds were chosen due to their possible antioxidant activities. The structures of all compounds were taken from National Institutes of Health (NIH) PubChem database and the research set was created. The conformational analysis and geometry optimizations were performed with Spartan’14 [9] via semi-empirical PM6 [10,11] and HF/6-31G(d) [12,13] methods, respectively. Single point ground state and time-dependent energies for antioxidant mechanisms were calculated with DFT//M06-2X level of the theory with 6-311+G(d,p) for gas phase and 6-31+G(d,p) basis sets for gas and water phases [14]. The value of H(H+), H(e-) and H(H.) in gas phase calculated with DFT//M06-2X/6-31+G(d,p) level of the theory and the values are 0.4963 au, -0.4491 au, -0.4966 au; in ethanol phase 0.4963 au, -0.7040 au, -0.4947 au, respectively, other side obtained results in gas phase with DFT//M06-2X/6-311+G(d,p) are 0.4963 au, -0.4979 au, -0.4981 au respectively.
Investigated Antioxidant Mechanisms
There are three important mechanisms in literature that are used for explaining the radical scavenging process of antioxidant molecules: Direct H Atom Transfer (HAT), Stepwise Electron Transfer-Proton Transfer (SET-PT) and Sequential Proton Loss Electron Transfer (SPLET). HAT mechanism’s main principle is homolytic O-H bond cleavage; H atom directly transfers from antioxidant (ArOH) to free radical (R.) (Rxn.1). The antioxidant capacity of the compound can be calculated by the Bond Dissociation Enthalpy (BDE) of the O-H bond. The lower BDE value shows that stability of the O-H bond is weak there with the antioxidant capacity of the compound is high.
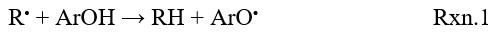
The second mechanism is SET-PT mechanism, which can be explained in two steps: electron cleavage from antioxidant (ArOH) and proton transfer from the radicalic cation (ArOH+•) (Rxn.2 Step 1). The Ionization Potential (IP) and Proton Dissociation Enthalpy (PDE) values are the most important factors for the activity of scavenging. Compounds with low IP and PDE values indicate the high scavenging activity.
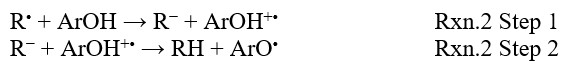
SPLET is the third mechanism, and it can be explained by three steps: formation of antioxidant anion and formation of flavonoid radical. These steps are governed by Proton Affinity (PA) and Electron Transfer Energy (ETE) values [15-18]
There are various parameters such as molecular descriptors for analyzing the antioxidant property of a compound; softness (S), hardness (η), electro negativity (χ), electrophilicity index (ω), electron affinity (A). These parameters are crucial for understanding the antioxidant activity of the compounds. η and χ values calculated by Parr and Pearson [19,20]. In this study, Koopman’s theory is used to determine ionization potential (I) and electron affinity values [21]. According to this theory, the negative Highest Occupied Molecular Orbital energy (-EHOMO) and the negative Lowest Unoccupied Molecular Orbital energy (-ELUMO) correlate to the ionization potential and electron affinity (i.e., I=-EHOMO and A=-ELUMO).
ω and S values calculated by the following equations:
BDE is related to the antioxidant properties, also lower values point out higher antioxidant activity of the corresponding compound [22]. It is observed in Table I that obtained BDE values were in general obtained smaller in polar environment by calculating 6-31+G(d,p) basis set with some exceptions, additional different basis sets did not give remarkable tendency on the energy values. The lowest BDE value is calculated for 3-BHA, 387.6 kJ mol-1 and 405.3 kJ mol-1 in gas and ethanol phase by the DFT//M06-2X/6-31+G(d,p), respectively.
Table 1. BDE and PDE values (kJ mol-1) of investigated molecules by M06-2X/6-31+G(d,p) and M06-2X/6-311+G(d,p) is gas and ethanol phases
Charged molecules are highly sensitive to the polarity of the solvent24. PDE values (in Table 1) obtained from the second step of the SET-PT mechanism have been determined in gas and ethanol phases, indicating that the PDE values have changed compared to environment polarity.
The polar solvent with approximately 130-200 kJ mol-1 significantly increase for PDE values when compared to gas phase; there are similar results in the literature [15,16]. The lowest PDE values (2162.1 kJ mol-1 and 2164.7 kJ mol-1; 2349.9 kJ mol-1, respectively) have been obtained for 1O position of the ascorbyl palmitate compared to other investigated compounds and their all studied OH positions in gas and ethanol phases. 2O position of the ascorbyl palmitate has almost the same values compared to 1O position, that meaning the furan ring of the molecule contributes to antioxidant activity of candidate molecule because of its aromatic property.
As for the calculated IP values calculated by the SET-PT mechanism both in gas and ethanol phases (in Table 2). The IP values in ethanol phase are approximately 800-850 kJ mol-1 lower than gas phase values. The found decreasing values of IP in condensed phase are in accordance with literature [15,23-25]. The lowest IP values were identified for 3-BHA structure in gas and 2-BHA in ethanol phases.
Table 2. IP values (kJ mol-1) of investigated molecules by DFT//M06-2X method
PA values of investigated compounds given in Table 3 via SPLET mechanism are ascorbyl palmitate (2O), it also has the lowest values compared to the other compounds, 2722.8 kJ mol-1 and 2500.0 kJ mol-1 in gas and ethanol phases, respectively. When investigated the calculated ETE values (in Table 3) via SPLET mechanism for modeled compounds, the values have been decreased approximately 300-400 kJ mol-1 in the condensed environment from in the gas phase. The obtained result is compatible with the results by Zheng et al and our previous studies in literature [15,23,24]. The lowest ETE values have been obtained for BHA molecules in the gas and water phases, which means it has the highest antioxidant property effective. The candidate sequence is as 2-BHA<3-BHA<ascorbyl palmitate(3O) in gas and ethanol phases by ETE values. The lower values of EHOMO and ELUMO band gap indicate the antioxidative capacity of the studied compound.
Table 3. PA and ETE (kJ mol-1) of investigated molecules by DFT//M06-2X method
The lower EHOMO shows weaker ability to proton, and higher EHOMO shows stronger e- donating ability. At the same time we have observed in our previous studies that the antioxidant activity has been found to be related to the distributions of frontier orbitals, as well [23,24]. The obtained frontier energy values and their distribution for the investigated molecules in the gas and ethanol solvents are depicted in Table 4 and Figure 2. The acquired results (Table 4) display that the antioxidant capability for the quercetin has the smallest band gap (EHOMO= -7.16 eV, ELUMO= -1.37 eV and band gap= -5.71 eV), therefore it is the most having antioxidant molecule according to band gap values. But we must emphasize that obtaining results from the distributions of the frontier orbitals are different. When the orbital distributions diffused to whole atoms of the molecule, antioxidant capability is decreased; yet the localized orbital distributions are more desirable to strong antioxidant capacity as shown in our published studies [25,26].
So other investigated molecules, ascorbyl palmitate, dodecyl gallate, 2-BHA and 3-BHA, are powerful candidates depending on orbital distributions although their band gaps are bigger than quercetin.
The molecular descriptors, which are η, S, ω, µ and χ values of the studied compounds, have been given in Table 4. Calculated χ, η, µ, S and ω molecular characteristics clearly demonstrate that the studied molecules prefer to act as e- donors instead of recipients in the studied phases [25,26].
Figure 2. The energies and distributions of HOMO and LUMO for investigated compounds
Table 4. Molecular descriptors of studied molecules with DFT//M06-2X method
All obtained the most important candidate molecules are given on Table 5 depend on investigated mechanism. Primary crucial molecule has been determined as BHA according to the BDE, ETE, IP and frontier orbital distributions. All essential mechanisms, which are essential to determine the antioxidant molecules, pointed out to BHA structure.
Table 5. Possible antioxidant molecules according to calculated parameters by different methods and phases
In this study we calculated the antioxidant properties of quercetin, kaempferol, ferulic acid, p-coumaric acid, BHA, ascorbyl palmitate and dodecyl gallate molecules. EHOMO/ELUMO, BDE, ETE, PA, IP and PDE values are calculated by using DFT//M06-2X method with 6-31+G(d,p) and 6-311+G(d,p) basis sets in the gas and ethanol phases. Both gas and ethanol phases were calculated with Spartan’14. We have modeled essential three mechanisms, HAT, SET-PT and SPLET, for investigating the antioxidant activity.
The obtained data showed that BHA family has superior antioxidant capacity compared to the other compounds according to investigated mechanisms and HOMO-LUMO distributions. The second crucial candidate is ascorbyl palmitate by PDE and PA values, but all especially 3-BHA demonstrated the lowest BDE values via using HAT mechanism and the highest EHOMO values. On the other hand, BDE values of 3-BHA decreased in polar solvent. The PDE and IP values that were calculated with SET-PT mechanism showed that ascorbyl palmitate had the lowest antioxidant ability compared to the other compounds. As expected, the phenolic ring compounds have higher electron donation ability than other molecules as quercetin, kaempferol and BHA. The lower values of band gap, magnitude difference between EHOMO and ELUMO values, indicate antioxidative capacity of the studied compounds and thus they can be ranged in the following order; quercetin, kaempferol, ferulic acid, p-coumaric acid, 2-BHA, 3-BHA, dodecyl gallate and ascorbyl palmitate. This clearly shows that BHA has the strongest H atom donating capability among the investigated compounds. In this study the relation between antioxidant activity and solvent effect for investigated compounds is demonstrated. Finally, we have shown that polar solvents strengthen electron donating of the majority of the molecules. The polar environment increased the PA values, which were calculated with SPLET mechanism.
The author (s) has no received any financial support for the research, authorship or publication of this study.
1 D.V. Ratnam, D. Ankola, V. Bhardwaj, D. Sahana & M.R. Kumar, Journal of Controlled Release 113(3) (2006) 189.
2 S. Li, H. Tan, N. Wang, Z. Zhang, L. Lao, C. Wong & Y. Feng, International Journal of Molecular Sciences 16(11) (2015) 26087.
3 K. Kawabata, R. Mukai, & A. Ishisaka, Food & Function 6(5) (2015) 1399.
4 D. Wu, J. Yan, P. Tang, S. Li, K. Xu, H. Li, Food Chem. 188 (2015) 370.
5 O. Bamidele, K. Duodu & M. Emmambux, Carbohydrate Polymers 166 (2017) 202.
6 C.A. Cordova, C. Locatelli, L.S. Assunção, B. Mattei, A. Mascarello, E. Winter, R.J. Nunes, R.A. Yunes, T.B. Creczynski-Pasa, Toxicology in Vitro 25(8) (2011) 2025.
7 N. Kumar & V. Pruthi, Biotechnology Reports 4 (2014) 86.
8 A. Sgarbossa, D. Giacomazza, M. Di Carlo, Nutrients 7 (2015) 5764.
9 W.J. Hehre. Wavefunction, Inc., Irvine, CA. (2003) 816p.
10 J.J. Stewart, J Mol. Model 14(6) (2008) 499.
11 J.J. Stewart, J Mol. Model 15(7) (2009 ) 765.
12 J.G. Valantin, Phys. Rev 122 (1961) 1012.
13 Y. Zhao and D.G. Truhlar, Theoretical Chemistry Accounts 120 (1-3) (2008) 215.
14 M. Yang, Z. Yu, S. Deng, X. Chen, L. Chen, Z. Guo, H. Zheng, L. Chen, D. Cai, B. Wen, Q. Wu & F. Liang, Scientific Reports 6(1) (2016).
15 A.V. Huravlev, G.A. Zakharov, B.F. Shchegolev & E.V. Savvateeva-Popova, PLOS Computational Biology 12(11) (2016).
16 G. Wang, Y. Xue, L. An, Y. Zheng, Y. Dou, L. Zhang & Y. Liu, Food Chemistry 171 (2015) 89.
17 H.H. Jaffe, J. Chem. Educ. 41(3) (1964) 172.
18 R.G. Pearson, Inorg. Chem. 27(4) (1988) 734.
19 R.C. Morrison, G.J. Liu, J Comput. Chem. 13 (1992) 1004.
20 Y. Huang, M. Lin, Y. Chao, C. Huang, Y. Tsai & P. Wu, International Journal of Urology 21(1) (2013) 94.
21 C.C.J. Roothaan , Rev. Mod. Phys. 23 (1951) 69
22 V.E. Atalay, Ocak H. SDU Fen Bil Enst Der. 23 (2019) 139.
23 F. De Vleeschouwer, V. Van Speybroeck, M. Waroquier, P. Geerlings & F. De Proft, Organic Letters 9(14) (2007) 2721.
24 T. Catal, S. Yavaser, V.E. Atalay, H. Bermek & S. Ozilhan, F. Ozen & T. Catal, Bioresource Technology 268 (2018) 116.
25 V.E. Atalay, B. Atasever-Arslan, B. Yaman, R. Cebecioglu, A. Kul, S. Ozilhan, F. Ozen & T. Catal, PLOS ONE 13(10) (2018).
26 V.E. Atalay, Y. Ayık, Chem. Pap. 73 (2019) 3105.
V.E. Atalay, I.S. Atish, K.F. Shahin, E.S. Kashikchi, M. Karahan, A DFT study: ranking of antioxidant activity of various candidate, molecules,UNEC J. Eng. Appl. Sci 2(1) (2022) 33-40
Anyone you share the following link with will be able to read this content:

This article is licensed under the Creative Commons Attribution ( CC BY 4.0 ) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
D.V. Ratnam, D. Ankola, V. Bhardwaj, D. Sahana & M.R. Kumar, Journal of Controlled Release 113(3) (2006) 189.
S. Li, H. Tan, N. Wang, Z. Zhang, L. Lao, C. Wong & Y. Feng, International Journal of Molecular Sciences 16(11) (2015) 26087.
K. Kawabata, R. Mukai, & A. Ishisaka, Food & Function 6(5) (2015) 1399.
D. Wu, J. Yan, P. Tang, S. Li, K. Xu, H. Li, Food Chem. 188 (2015) 370.
O. Bamidele, K. Duodu & M. Emmambux, Carbohydrate Polymers 166 (2017) 202.
C.A. Cordova, C. Locatelli, L.S. Assunção, B. Mattei, A. Mascarello, E. Winter, R.J. Nunes, R.A. Yunes, T.B. Creczynski-Pasa, Toxicology in Vitro 25(8) (2011) 2025.
N. Kumar & V. Pruthi, Biotechnology Reports 4 (2014) 86.
A. Sgarbossa, D. Giacomazza, M. Di Carlo, Nutrients 7 (2015) 5764.
W.J. Hehre. Wavefunction, Inc., Irvine, CA. (2003) 816p.
J.J. Stewart, J Mol. Model 14(6) (2008) 499.
J.J. Stewart, J Mol. Model 15(7) (2009 ) 765.
J.G. Valantin, Phys. Rev 122 (1961) 1012.
Y. Zhao and D.G. Truhlar, Theoretical Chemistry Accounts 120 (1-3) (2008) 215.
M. Yang, Z. Yu, S. Deng, X. Chen, L. Chen, Z. Guo, H. Zheng, L. Chen, D. Cai, B. Wen, Q. Wu & F. Liang, Scientific Reports 6(1) (2016).
A.V. Huravlev, G.A. Zakharov, B.F. Shchegolev & E.V. Savvateeva-Popova, PLOS Computational Biology 12(11) (2016).
G. Wang, Y. Xue, L. An, Y. Zheng, Y. Dou, L. Zhang & Y. Liu, Food Chemistry 171 (2015) 89.
H.H. Jaffe, J. Chem. Educ. 41(3) (1964) 172.
R.G. Pearson, Inorg. Chem. 27(4) (1988) 734.
R.C. Morrison, G.J. Liu, J Comput. Chem. 13 (1992) 1004.
Y. Huang, M. Lin, Y. Chao, C. Huang, Y. Tsai & P. Wu, International Journal of Urology 21(1) (2013) 94.
C.C.J. Roothaan , Rev. Mod. Phys. 23 (1951) 69
V.E. Atalay, Ocak H. SDU Fen Bil Enst Der. 23 (2019) 139.
F. De Vleeschouwer, V. Van Speybroeck, M. Waroquier, P. Geerlings & F. De Proft, Organic Letters 9(14) (2007) 2721.
T. Catal, S. Yavaser, V.E. Atalay, H. Bermek & S. Ozilhan, F. Ozen & T. Catal, Bioresource Technology 268 (2018) 116.
V.E. Atalay, B. Atasever-Arslan, B. Yaman, R. Cebecioglu, A. Kul, S. Ozilhan, F. Ozen & T. Catal, PLOS ONE 13(10) (2018).
V.E. Atalay, Y. Ayık, Chem. Pap. 73 (2019) 3105.